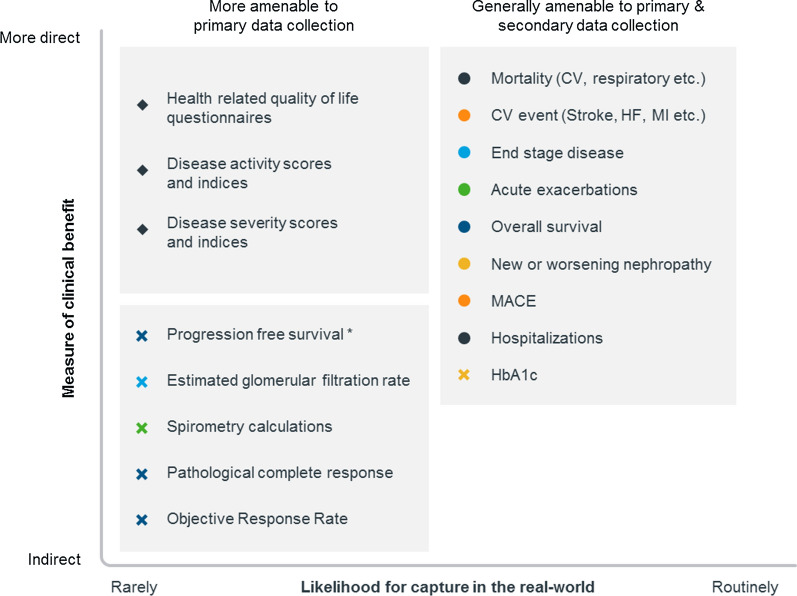
Figure 1.
The Assessment of Clinical Trial Endpoints Along the Axes of Real-World Availability and Measure of Clinical Benefit. Common endpoints examined from cardiovascular disease (orange), chronic kidney disease (light blue), diabetes (yellow), oncology (dark blue), respiratory (green), and general disease assessments (black). Types of endpoint illustrated include: direct or clinical (circles), surrogate (crosses), and patient-centric (diamonds). CV Cardiovascular, MACE major adverse cardiovascular event, HbA1c haemoglobin A1c, HF heart failure, MI myocardial infarction. *Progression free survival when measured by RECIST