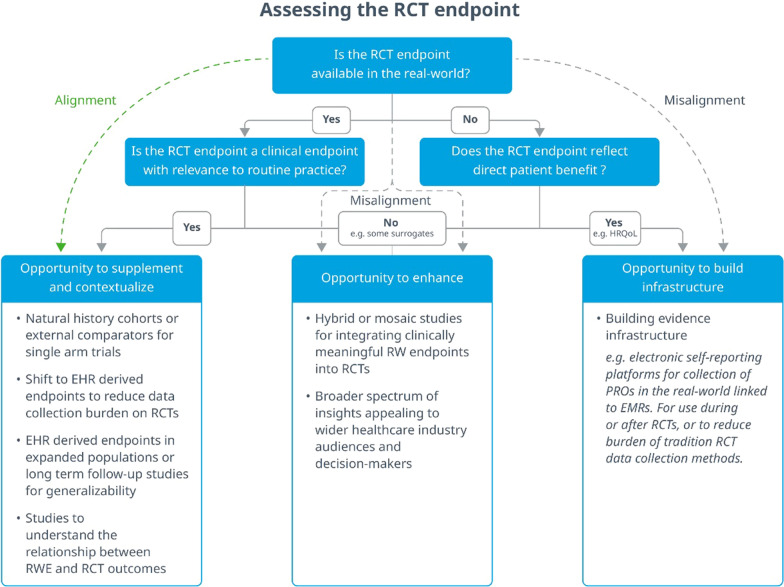
Figure 2.
A Framework Proposing Several Pathways to Integrating RWE in the RCT Setting Based on the Clinical Relevance and Real-World Availability of Trial Endpoints. Trial endpoints that are relevant to routine practices, show a clinical benefit, and available in the real world represent an alignment with real-world study endpoints, and these RCTs could benefit from RWE supplementation or contextualisation. However, clinical trial endpoints that are not available in the real world or that do not measure direct clinical outcomes, or both, represent a misalignment with RWE and can benefit from hybrid methodologies or building real-world infrastructure