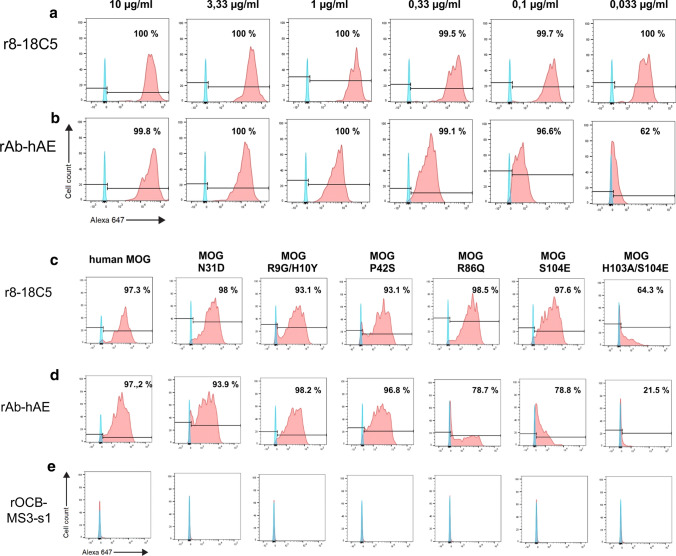
Fig. 5.
Recognition of hMOG. a, b COS-7 cells were transiently transfected with full length hMOG and analyzed by flow cytometry for hMOG-binding of rAb-hAE. The cells were stained with the control antibody r8-18C5 (a) and rAb-hAE (b) at concentrations ranging from 10 to 0.033 µg/ml. The percentages of positive cells are indicated in the plots. Both antibodies recognized hMOG but the reduced shift of rAb-hAE below 1 µg/ml indicated a lower affinity for hMOG by rAb-hAE as compared to r8-18-C5. To determine the epitope of rAB-hAE, COS-7 cells were transiently transfected with full length hMOG or hMOG-mutants and analyzed for recognition of r8-18C5 (c), rAb-hAE (d), and the negative control antibody rOCB-MS3-s1 (e) by flow cytometry. For each sample, 30 µg/ml antibody were used. The amino acid substitutions are indicated at the top and the percentages of positive cells are indicated in the plots. Substitutions of histidine 103 and serine 104 by alanine and glutamine acid showed a strong decrease of hMOG-binding for rAb-hAE, proving that these amino acids are the dominant part of the epitope. The single substitutions S104E and R86Q had minor effects only on rAb-hAE but not on r8-18C5