Abstract
Prenatal infections have long been recognized as important, preventable causes of developmental disabilities. The list of pathogens that are recognized to have deleterious effects on fetal brain development continues to grow, most recently with the association between Zika virus (ZIKV) and microcephaly. To answer clinical questions in real time about the impact of a novel infection on developmental disabilities, an historical framework is key. The lessons learned from three historically important pathogens: rubella, cytomegalovirus, and ZIKV, and how these lessons are useful to approach emerging congenital infections are discussed in this review. Congenital infections are preventable causes of developmental disabilities and several public health approaches may be used to prevent prenatal infection. When they cannot be prevented, the sequelae of prenatal infection may be treatable.
When considering the underlying etiology of developmental disabilities, it is essential to recognize the complex interplay between developmental biology and the environment, particularly during critical periods of early brain development. In this regard, prenatal infections represent a large and important group of causes of developmental disabilities, variably resulting in infants and children with intellectual and learning disabilities, hearing impairment, vision impairment, cerebral palsy (CP), autism spectrum disorder, and disorders of language, attention, and behavior. While the five major ‘TORCH’ infections (toxoplasmosis, syphilis, rubella, cytomegalovirus, herpes) have been traditionally recognized as the most common, there are a growing number of other pathogens of epidemiological importance, particularly in the post-vaccine era (Table 1). However, this list of congenital infections is not stagnant. In the realm of infectious diseases, new pathogens unexpectedly emerge, posing new questions and presenting challenges to the understanding of prenatal infection. This has most recently been seen with the sudden appearance of increased rates of congenital microcephaly in the setting of the outbreak of Zika infection.1
Table 1:
Prenatal infections associated with neurodevelopmental disabilities
| Toxoplasmosis |
| Syphilis |
| Varicella |
| Parvovirus B19 |
| Rubella |
| Cytomegalovirus |
| Herpes simplex virus |
| Congenital lymphocytic choriomeningitis syndrome |
| Human immunodeficiency virus |
| West Nile virus |
| Chikungunya |
| Coxsackie virus |
| Hepatitis virus |
| Zika virus |
Within this growing group of pathogens, rubella, cytomegalovirus, and Zika virus (ZIKV) are historically important prenatal infections which can lead to fetal loss, growth restriction, and developmental disorders. Each is a vertically transmitted pathogen that illustrates concepts key to understanding the ongoing and future clinical implications of prenatal infections as preventable causes of developmental disabilities. In this review, we highlight the clinical and neuroscientific lessons learned from these pathogens and how these lessons can be used to approach novel or emerging infections. (For a comprehensive review of additional prenatal infections, see other key references cited below.2,3)
PROTECTIVE MATERNAL–FETAL BARRIERS: WHY DO NOT ALL INFECTIONS CAUSE DEVELOPMENTAL DISABILITIES?
Females frequently experience infections during pregnancy, ranging from the common cold to urinary tract infections and severe viral illnesses. Most infections are well-tolerated without detrimental effects on the fetus, resulting in typical infant outcomes. The mechanism by which some of such pathogens impact development has been clearly documented. However, it is also important to emphasize that while maternal infection by other pathogens, such as influenza, may increase the risk of preterm birth, they do not appear to have direct harmful effects on development.4-6 So, why do not all maternal infections lead to problems with fetal development and developmental disabilities? It is possible that the key to the most deleterious fetal outcomes depend on the capability of selected pathogens to invade the fetal environment and produce direct pathogenic effects on developing fetal systems.
The barriers preventing fetal infection are critical to the development of a ‘healthy’ child. The two key barriers in this regard are the placental-fetal barrier and the fetal blood brain barrier (BBB), which separates the fetal systemic circulation from the central nervous system (CNS). Those barriers are the doorkeepers and roadblocks to pathogen trafficking into the fetal environment. Only a small number of infectious agents are able to cross the placenta, a measure of the importance of the placenta in preventing fetal exposure to pathogens, and then cross the BBB into the CNS. The fetal and maternal immune systems, through intricate molecular signaling pathways and diverse cell types, play important roles in protecting the fetus from a wide range of potentially deleterious exposures, which can disrupt the spatially and temporally orchestrated development of the CNS.
The placental–fetal barrier
The placenta is the key barrier between the maternal and fetal circulation, protecting the fetus from pathogens, toxicants, and teratogens as well as providing metabolite exchange, allowing maternal antibodies into the fetal circulation, hormone production, and regulating nutrition and waste removal via placental syncytiotrophoblasts. One half is of fetal origin and the other half of maternal origin. One of the many remarkable characteristics of the placenta is that it must simultaneously serve as a barrier against a wide range of pathogens, while at the same time not rejecting the fetus, a semi-allograft with ‘foreign’ antigens.7
This barrier is focused at the maternal–fetal interface, which comprises systemic and local immunity mechanisms protecting both the developing fetus and the mother. Such immune mechanisms facilitate the interplay of placental cellular systems and immune mediators and receptors to maintain the fetal homeostasis. Immune signaling pathways that facilitate pathogen recognition, Toll-like and Nod-like receptors, along with the network of cytokines and chemokines that modulate immune cell reactions of natural killer cells, Hofbauer cells, T cells, and dendritic cells as well as mast cells, B cells, and innate lymphoid cells, act together to identify and respond to the challenge of pathogens and maternal infections.8
The fetal BBB
Within the fetus, the BBB is a complex, consisting of a continuous non-fenestrating microvasculature in combination with support provided by mural, immune, glial, and neural cells to protect the brain from infection or toxins.9 This complex, termed the neurovascular unit, tightly regulates the movement of ions, molecules, and cells in and out of the CNS. The BBB develops as early as 8 weeks gestation, but how its function evolves in unison with brain maturation is not fully understood.10 Although the CNS is well-sealed and protected from environmental challenges, a well-established trafficking of cells from the immune system (e.g., lymphocytes) and antibodies is regulated by the BBB to facilitate the immune surveillance of the system and respond to potential infections of the CNS.
When these mechanisms fail
During the last two decades there have been great advances in understanding of the pathophysiology of viral infections and how they can evade host detection. While the pathophysiology is not fully understood, to cause fetal infection, viruses must be bloodborne in sufficient viral quantities to produce infection by interaction with specific receptors and hijack cellular mechanisms for viral replication and production of specific viral proteins with cytotoxic properties.8
For cytomegalovirus and ZIKV the mechanisms may vary. In primary cytomegalovirus infection during the first trimester of pregnancy, there is a 40% risk of direct transmission to the fetus.8 After initial infection the virus establishes lifelong lineage in monocytes, the proper cell for viral replication. While specific details are not known, in recurrent congenital infection, the virus is activated in the placental environment and passes through to the fetus. Similarly, ZIKV has the capability of infecting monocytes and subsequently targeting placental cells via the bloodstream. One possible mechanism of transplacental passage is via infected monocytes through trans-endothelial migration. In the fetal environment, ZIKV has a high neurotropic and neurovirulent capability producing fetal encephalitis. Additionally, ZIKV infection can trigger cascades of proinflammatory cytokines leading to tissue injury.11
CONGENITAL RUBELLA SYNDROME: EARLY INSIGHTS AND PUBLIC HEALTH SUCCESS
Congenital rubella syndrome (CRS) represents one of the first congenital infections to be linked to developmental disabilities and the first to be eradicated – serving as a model for which subsequent efforts against prenatal infections have been compared.
At the beginning of the 20th century, rubella was thought to be a benign illness from which most people recovered. However, in 1941 Sir Norman Gregg, an Australian ophthalmologist, reported cataracts, deafness, and congenital heart disease in infants whose mothers contracted the German measles.12 This association between maternal infection and neonatal outcome was the first to recognize this seemingly benign viral infection as a potentially devastating threat during pregnancy. In the next several decades, as cases continued to occur, the phenotype of CRS was expanded to include microcephaly and developmental disability. CRS is now described by four major criteria, which include cataracts or congenital glaucoma, congenital heart disease, hearing impairment, and pigmentary retinopathy and several minor criteria with developmental consequences. Long term, while the literature on the developmental outcomes of individuals with CRS is limited, one study of 53 adolescents born during the 1965 epidemic reported hearing impairment in 92%, vision impairment in 56%, and CP in 19%. Intellectual disability was reported in approximately 28% of individuals with CRS, the majority of whom had severe to profound intellectual disability.13 In a more recent study of individuals with CRS in Oman, 33% died during infancy; in those who survived, 84% had hearing impairment, 84% had vision impairment, 53% had intellectual disability, 42% had CP, and 2% had epilepsy.14 The case definition for CRS, outlining the spectrum of clinical manifestations, is described in Table 2.15
Table 2:
Case definition for congenital rubella syndrome
| Suspected | Probable | Confirmed |
|---|---|---|
An infant who does not meet the criteria for a probable or confirmed case but who has one or more of the following findings:
|
An infant who does not have laboratory confirmation of rubella infection but has at least two of the following, without a more plausible etiology:
one of the above AND one or more of the following:
|
An infant with at least one of the symptoms clinically consistent with congenital rubella syndrome listed to the left, and laboratory evidence of congenital rubella infection demonstrated by one or more of the following findings:
|
In the 1960s, an epidemic of rubella swept across Europe and the United States and approximately 20 000 infants were born with CRS.16 This unfortunate rise in cases led to the important recognition that timing of infection during specific periods of vulnerability impacts outcome. It was observed that the earlier the timing of infection during pregnancy, the more likely the infant would present with CRS and the more severe the manifestations. With the scientific advances of serological testing to confirm infection, the association between timing of infection and outcome was more clearly delineated, identifying a critical period of vulnerability during the first trimester of gestation.17 One seminal study prospectively followed over 1000 females with confirmed rubella infection in different stages of pregnancy. When they followed infants until 2 years of age, 100% of infants infected before the 11th week had congenital heart disease and deafness, 35% of those infected between 13 and 15 weeks had deafness alone, and no defects were found in those children exposed after 16 weeks.18
In 1969, immunization against rubella was introduced, leading to the near complete elimination of this infection in developed countries over succeeding decades.12,16 The rubella vaccine is included in the routine childhood immunization schedule in the combined measles mumps rubella vaccination at ages 12 months and 4 years. Therefore, most females of childbearing age in developed countries are now immune to rubella before becoming pregnant. Herd immunity has also resulted in absent transmission of the rubella virus in the United States since 2012. Currently the Center for Disease Control and Prevention reports less than one case of CRS per year in the United States and infections are associated with travel.19 Because of these astounding immunization and eradication efforts, many newly trained physicians will never see a case of CRS. However, this public health success story remains relevant as rubella continues to circulate in countries with poor healthcare resources. Further, the threat of reemergence of rubella in developed countries due to anti-vaccine movements and declining immunization rates is of increasing concern.
Ironically, because of the near eradication of rubella and absence of an animal model, relatively little is known about the pathophysiological mechanisms by which the rubella virus impacts on fetal brain development. Histopathological studies of aborted fetuses and deceased newborns during the epidemic of the 1960s were limited to lesions in several organ systems consistent with the clinical manifestations of CRS.20,21 In the brain, studies using human cell culture have demonstrated that the rubella virus preferentially invades astrocytes in vitro and spares oligodendrocytes.22 A more recent study from 2016 used immunohistochemistry to identify viral antigens in three fatal cases of neonates with CRS. Notably, rubella virus was present in two major cell types: the progenitor cell of the granular layer of the cerebral cortex as well as in myocardial fibroblasts.23 This identification of rubella viral particles in this specific area of the brain suggests a potential explanation for microcephaly, neurological impairment, and developmental disabilities that is seen in people with CRS. Similar findings have also been identified in fetal ciliary bodies, probably representing precursors to the ocular findings of CRS. 24 Two mechanisms have been suggested for rubella virus teratogenicity: rubella-virus-induced cell death by apoptosis and rubella-virus-induced inhibition of mitosis by blocking the assembly of actin.25 Finally, the completion of organogenesis and the development of the fetal immune system are postulated to play a role in the observation that timing of infection determines outcome, but further evidence is needed to support these hypotheses.25
CONGENITAL CYTOMEGALOVIRUS: A MODERN CHALLENGE
Congenital cytomegalovirus has long been recognized as an important cause of developmental disabilities. However, in contrast to rubella, cytomegalovirus has been challenging to prevent and treat and remains a persistent congenital infection today, in both developed and developing countries. Whereas rubella virus and ZIKV, both RNA viruses, produce limited febrile illnesses, cytomegalovirus, a DNA virus, has the capability to produce long-term asymptomatic infection with the potential for reactivation. Notably, almost 50% of the population is already infected with cytomegalovirus by age 40 years.
Reports of cases highlighting the findings associated with congenital cytomegalovirus were described as early as the 1880s, but it was only in 1956, when Birdsong et al. first made the connection to a viral infection in a series of neonates who died shortly after birth and were found to have viral inclusions in their brain tissues.26 Today, congenital cytomegalovirus remains the most common congenital infection, affecting 0.2% to 0.7% of live births in the United States.27,28 Non-white ethnicity and low socio-economic status are risk factors for congenital cytomegalovirus, highlighting the impact of healthcare disparities on prenatal infections.29
In infants with congenital cytomegalovirus, 10% are symptomatic at birth and may manifest with low birthweight, microcephaly, petechiae, hepatosplenomegaly, and hearing loss. Fifty to 75% of these infants will have long-term developmental disabilities, including cognitive deficits, motor deficits, hearing deficits, and epilepsy (Table 3).28,30-32 In one study of outcomes of 170 infants with symptomatic congenital cytomegalovirus, approximately one third had neurological impairment at follow-up and of those, 62% had severe intellectual impairment.32 Other longitudinal studies of infants with symptomatic congenital cytomegalovirus reported CP in approximately 30% to 50% of the children, the majority of whom also had intellectual disability.30,31 Notably, the most important predictor of poor developmental outcome in symptomatic congenital cytomegalovirus was microcephaly at birth.30,31
Table 3:
Potential developmental disabilities associated with congenital infection
| Immediately evident at birth | Microcephaly, epilepsy, petechiae, purpura, jaundice, hepatosplenomegaly, hydrops, low birthweight |
| Evident after investigation | Sensorineural hearing loss, retinitis, intracranial calcifications, hydrocephalus, thrombocytopenia |
| Evident long-term | Cerebral palsy, intellectual disability, learning disability, attention-deficit/hyperactivity disorder, delayed-onset hearing loss, epilepsy, feeding disorders |
Of the 90% of infants who are asymptomatic at birth, 15% to 25% will go on to have sensorineural hearing loss.27 These children who are asymptomatic at birth typically have intelligence and academic performance similar to population norms.33-35 This realization of delayed-onset hearing loss highlights that this (and other) congenital infections may have more subtle consequences that are not evident at birth and may require investigation later in childhood. Long-term outcomes of children with congenital cytomegalovirus continue to be an active area for research.
Similarly to rubella, there is evidence for cytomegalovirus that timing of infection during pregnancy has an impact on outcome. Interestingly, while intrauterine transmission rates are highest during the third trimester, the likelihood of the neonate being symptomatic at birth is highest during the first trimester.36-39 One study of over 200 pregnancies with confirmed primary cytomegalovirus infection, detected intrauterine transmission by amniocentesis in 30%, 38%, and 72% of cases during the first, second, and third trimester respectively. However, rates of symptomatic infection were 28%, 14%, and 0%, in the first, second, and third trimesters respectively; infants with third trimester infection had typical hearing and development at follow-up.39
What has been most challenging to the treatment and prevention of cytomegalovirus is that immunocompetent adults are typically asymptomatic, and the mean age of seroconversion is 28 years, coinciding with peak childbearing years in females.40 Primary infection (infection for the first time) during pregnancy has been most frequently associated with congenital infection, but secondary infection (a subsequent instance of infection) with novel serotypes may also be causative.
Vaccine development efforts are considered a priority by various medical and scientific organizations and several candidate vaccines are under investigation.41 However, to date there is no vaccine against cytomegalovirus on the market. A 2009 phase two study of a vaccine consisting of recombinant cytomegalovirus envelope glycoprotein in females of childbearing age yielded only 50% efficacy and one congenital infection occurred in the vaccine group.42 Current ongoing clinical trials focus on novel vaccine development in patients undergoing hematopoietic stem cell transplant.43 Challenges to the development of an effective vaccine against cytomegalovirus include lack of an animal model and only recent emphasis on the importance of cellular immunity over neutralizing antibodies for protection against this virus.41
In the absence of a vaccine to prevent infection, efforts against cytomegalovirus have employed other strategies for intervention. Antivirals have been explored as a method to mitigate consequences in those who have been infected. A study of oral valganciclovir in symptomatic infants during the first 6 months of life reported improved hearing and developmental outcomes at 2 years of age.44 There are also ongoing clinical trials investigating whether antivirals or passive immunization with cytomegalovirus hyperimmune globulin during pregnancy may improve outcome.45
While the underlying pathophysiology for cytomegalovirus disease in adults has been studied, the primary mechanisms disrupting fetal brain development are complex and less well understood. Specifically, research on the pathogenesis of this congenital infection has been limited because of a lack of adequate animal models. It is postulated that gene products of the virus interfere with normal cellular activity, inducing apoptosis, cellular proliferation, inflammation, and other processes that are critical to typical fetal development.46 However, exciting new advances in neuroscience technology, including the creation of human brain organoid models, may facilitate basic science research on congenital cytomegalovirus and may lead to potential therapeutic interventions over the next decade.
Finally, newborn screening for cytomegalovirus is an area of active study and heated debate. Proposed methods of screening include testing infants who fail the newborn hearing screen or testing all newborns. The state of Utah implemented the former, termed ‘hearing targeted’ approach in 2013.47,48 Results of antiviral clinical trials may impact whether similar public health policies are instituted in the future. If implemented, cytomegalovirus would be the first congenital infection to be included in the universal newborn screen.
CONGENITAL ZIKA SYNDROME: A NEWLY RECOGNIZED PATHOGEN
The list of infections with deleterious effects on fetal development continues to grow. New congenital infections are primarily recognized in two ways: through case series of rare prenatal infections with longitudinal follow-up, or through increased epidemiological case recognition in the context of new outbreaks or epidemics. The latter has most recently occurred with the emergence of ZIKV infection outbreaks in the Americas in 2015/2016. The story of ZIKV as a prenatal infection is an example of how pathogens that cause developmental disabilities may arise at any time and the lessons learned from other congenital infections are key to clinical and public health response. It also illustrates how modern scientific technologies and global communication networks have facilitated rapid research and understanding of the pathophysiology of this developmental disability.
The ZIKV was first reported in 1947 in the Zika Forest in Uganda and remained a fairly benign mosquito-borne illness in Africa and Asia until 2007 when a large outbreak was reported in Yap Island, Micronesia and 2013 when a second outbreak was reported in French Polynesia.49 However, no connection was made to birth defects until 2016 when an epidemic in Brazil coincided with an increase in cases of infants born with profound microcephaly.50
Extensive global public health and clinical research efforts have led to rapid understanding of the clinical spectrum and pathogenesis of congenital Zika syndrome (CZS). ZIKV is an RNA-virus primarily transmitted by mosquito bites, but also recognized to have the potential for sexual transmission. ZIKV infection during pregnancy is associated with failed neuronal migration and neuronal cell death, resulting in fetal brain disruption sequence. Fetal brain disruption sequence manifests as five major structural anomalies: severe microcephaly with collapsed skull, cortical thinning of the brain (with calcifications and abnormal gyral patterns), eye anomalies, contractures, and hypertonia. Deafness is also common. Although other congenital infections may present with overlapping features, this constellation of findings together seems to be unique to CZS.51 The cranial involvement in CZS manifests as microcephaly and overlapping cranial sutures. The spectrum of brain involvement in CZS may include a variable magnitude of encephalitis which evolve into arrest of cortical development with cortical thinning or abnormal gyration, intracranial calcifications, hydrocephalus, congenital contractures, eye anomalies, and deafness.52 Infants affected by CZS have thus far shown severe developmental delays and are at high risk for lifelong developmental disabilities.1,53,54 However, given that ZIKV as a congenital infection has only been recently recognized, the infants affected by this virus have yet to reach adolescence or adulthood and many questions about the long-term implications of ZIKV infection remain unanswered. One study of 121 infants with CZS in Brazil reported profound developmental delays at 2 years 6 months of age. Nearly all infants in this cohort performed at 2 to 4 months age equivalent across all functional domains on the Bayley Scales of Infant Development.54
Similar to cytomegalovirus, it has been recognized that even those infants with prenatal ZIKV exposure who appear ‘normal’ at birth may go on to exhibit more subtle developmental disabilities later on.55 Although children born with normal head circumference after in utero ZIKV exposure exhibited fewer neurological abnormalities than children born with microcephaly, a longitudinal follow-up at 6 months to 3 years 6 months of a cohort of 109 children with normal head circumference born by mothers infected by ZIKV, exhibited frequent neurological abnormalities (68%) which included signs of motor dysfunction on neurological examination, feeding problems, and abnormal brain imaging.56 Interestingly, the majority (64%) of this cohort fell within the average or above average range on developmental assessment. In both infants with CZS and those with normal head circumference, the strongest predictor of developmental outcome in infants with prenatal exposure to ZIKV is head circumference at birth, with smaller heads predicting poorer developmental performance.54,56 Future longitudinal studies of this population will further elucidate the clinical phenotype of these children.
New knowledge about ZIKV draws many parallels to rubella and cytomegalovirus. Similarly to cytomegalovirus, ZIKV infection is asymptomatic in 80% of individuals and is, therefore, a challenge to detect. However, in contrast to other congenital infections, transmission of ZIKV by an insect poses different strategies for infection prevention (e.g. travel advisories, insect repellants, long clothing). Again, timing of infection with ZIKV impacts on outcome, with infants exposed during the first trimester having increased likelihood of fulminant CZS compared to those exposed later. Females with low socio-economic status are also at higher risk.57
Despite the relatively recent emergence of Zika infection, effective animal and organoid models have facilitated significant strides toward the understanding of the underlying pathogenesis that impacts on fetal brain development. Mouse models have demonstrated not only that ZIKV can cross the placenta and invade fetal tissue after inoculation of a pregnant dam, but also that ZIKV preferentially invades and replicates in fetal brain tissue over other organs, specifically neural progenitor cells.58,59 ZIKV has also been shown to subsequently interfere with the proliferation of neural tissue, leading to cell death, disrupted cortical layers, and reduced brain volume in both mouse and human brain organoid models.60,61 Other studies have implicated the centrosome, where ZIKV infection triggers centrosome disruption leading to unequal neural progenitor cell division, premature differentiation, depletion of progenitor cell numbers, and cortical thinning.60 Epigenetic changes may also be at play. One study demonstrated that ZIKV infection alters the topology of both human and viral RNA by influencing methylation patterns to facilitate ZIKV replication.61
Will ZIKV remain a persistent threat, or was this a fleeting pandemic? The answer remains unclear. While new cases of ZIKV infection have dramatically declined since 2016, in a large part due to herd immunity, it is possible that the virus will reemerge or remain endemic in areas of the world with potential for mosquito transmission. Nevertheless, its importance as a newly recognized congenital infection associated with the emergence of pandemic remains evident.
THEMES AND CLINICAL IMPLICATIONS
Rubella, cytomegalovirus, and ZIKV represent important prenatal infections that have elucidated several themes with significant clinical implications.
Theme 1: There is frequently a striking juxtaposition between the severity of illness in the mother and outcome of the infant
In all the prenatal infections discussed above, the pathogen was initially considered benign because the manifestations of illness in adults or children were mild or absent. However, these pathogens may preferentially affect the cells of the developing fetus, leading to severe developmental disability. When considering prenatal infection as the etiology of an infant or child with a developmental disability, the absence of a history of maternal symptoms does not preclude this diagnosis. Instead, gathering history about risk factors for infectious exposure may be more pertinent.
Theme 2: Timing matters
The physical manifestations of prenatal infection correlate with the time during gestation that the infection was acquired. Infections early in pregnancy, particularly during organogenesis of the first trimester, may lead to more severe outcomes, frequently associated with structural anomalies. In contrast, infections later in pregnancy may result in more subtle manifestations. However, these subtle features (e.g. deafness or learning disability) may, nonetheless, have an important impact on the life of the child.
Theme 3: These are preventable causes of developmental disabilities
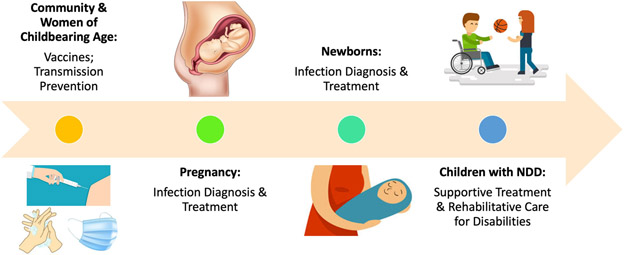
Infections can frequently be prevented and therefore, these forms of congenital infection can also be prevented. There are several approaches to the prevention of prenatal infection (Fig. 1). Universal immunization and herd immunity are the most effective strategies for eradication. However, in the absence of an available vaccine, handwashing, personal protective equipment, and travel restrictions for vulnerable populations can be effective. Specifically, for diseases transmitted by animals, vector control may also be useful.
Figure 1:
Opportunities for intervention at different points in the timeline of prenatal infections associated with neurodevelopmental disabilities (NDD). (Illustration courtesy of royalty-free stock images on dreamstime.com).
Theme 4: When it cannot be prevented, congenital infection is treatable
While the efficacy of interventions during pregnancy are still being explored, there are many opportunities to mitigate disability due to congenital infection during the neonatal period and into childhood (Fig. 1). For many of these interventions, early implementation is associated with better outcome. Specifically, while antivirals are still under active investigation, many current therapeutic approaches are known to be effective. For example, infants with sensorineural hearing loss may be candidates for hearing augmentation or cochlear implants62 and infants with developmental delay benefit from early intervention services.63 Furthermore, knowledge of the cause of a developmental disability can be important to assess the risk to future pregnancies.
Theme 5: Because they may be treatable, diagnostic testing is important to identify cases
Of note, during the critical period of opportunity to mitigate disability, many patients are asymptomatic. For this reason, good diagnostic testing coupled with surveillance or screening programs to identify which individuals are at risk is critical. Whether this is done through surveillance or screening of all pregnant females or all infants remains to be determined.
PERSPECTIVE ON DEVELOPMENTAL DISABILITIES AND EMERGING INFECTIONS
Most recently, the COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has affected all segments of the world’s population, and raised concerns about fetal risk. Although this story is still evolving, the risk of passing the infection to the fetus appears to be very low. Furthermore, neurological complications of SARS-CoV-2 appear to be associated with secondary systemic inflammation or hypoxia rather than a neurovirulent effect of the virus.64,65 At present, SARS-CoV-2 has not been demonstrated to be neurotropic or neurovirulent in adults. In one study of 55 pregnant females infected with SARS-CoV-2 and 46 neonates, there was no definite evidence of vertical transmission.66 Another case series of 33 pregnancies identified SARS-CoV-29 infection in three newborns, but it is unclear if infection occurred in the womb or after birth.67 It is possible that maternal infection and the aftermath of infection, such as inflammatory responses, eventually may influence neurodevelopmental trajectories of the fetus. Longitudinal data will be needed to fully understand the impact of this novel virus on fetal outcomes. The principles discussed above will help guide clinical judgement and research development as the story unfolds.
CONCLUSION
Prenatal infections are a dynamic and solvable problem. History has provided valuable insights that have influenced the current and future management of prenatal infections. These guiding principles are important not only for families to understand their child, but also to devise public health and research strategies for prevention or treatment. Significant strides have already been made to expand the understanding of how infections impact brain function. Moving forward, we hope that like rubella, the next generation will witness the eradication of some infectious causes of developmental disabilities.
What this paper adds.
The list of prenatal infections associated with developmental disabilities continues to increase.
Lessons learned from rubella, cytomegalovirus, and Zika virus have implications for new pathogens.
Severity of illness in the mother does not correlate with severity of sequelae in the infant.
Acknowledgements
The authors wish to acknowledge Paul Lipkin, Mary Leppert, Maria Alejandra Garcia- Dominguez, and Elaine Stashinko for their thoughtful insights on this manuscript. Carlos A Pardo is supported by the Bart McLean Fund for Neuroimmune Research and NIH (R01-NS110122). The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
ABBREVIATIONS
- BBB
Blood brain barrier
- CRS
Congenital rubella syndrome
- CZS
Congenital Zika syndrome
- ZIKV
Zika virus
References
- 1.Gordon-Lipkin E, Peacock G. The spectrum of developmental disability with zika exposure: what is known, what is unknown, and implications for clinicians. J Dev Behav Pediatr 2019; 40: 387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meaney-Delman D, Jamieson DJ, Rasmussen SA. Addressing the effects of established and emerging infections during pregnancy. Birth Defects Res 2017; 109: 307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Accardo PJ, Capute AJ. Capute & Accardo’s Neurodevelopmental Disabilities in Infancy and Childhood: Neurodevelopmental Diagnosis and Treatment. Baltimore: Brookes Publishing, 2008. [Google Scholar]
- 4.Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012; 130: e1447–e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerbo O, Qian Y, Yoshida C, Fireman BH, Klein NP, Croen LA. Association between influenza infection and vaccination during pregnancy and risk of autism spectrum disorder. JAMA Pediatr 2017; 171: e163609. [DOI] [PubMed] [Google Scholar]
- 6.Zerbo O, Iosif A-M, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord 2013; 43: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong M, Abrahams VM. Immunology of the Placenta. Obstet Gynecol Clin North Am 2020; 47: 49–63. [DOI] [PubMed] [Google Scholar]
- 8.Pereira L Congenital viral infection: Traversing the uterine-placental interface. Annu Rev Virol 2018; 5: 273–99. [DOI] [PubMed] [Google Scholar]
- 9.Daneman R, Prat A. The Blood–Brain Barrier. Cold Spring Harb Perspect Biol 2015; 7: a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goasdoué K, Miller SM, Colditz PB, Björkman ST. Review: The blood-brain barrier; protecting the developing fetal brain. Placenta 2017; 54: 111–6. [DOI] [PubMed] [Google Scholar]
- 11.Khaiboullina SF, Ribeiro FM, Uppal T, Martynova EV, Rizvanov AA, Verma SC. Zika virus transmission through blood tissue barriers. Front Microbiol 2019; 10: 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plotkin SA. The history of rubella and rubella vaccination leading to elimination. Clin Infect Dis 2006; 43 (Suppl 3): S164–S168. [DOI] [PubMed] [Google Scholar]
- 13.Desmond MM, Wilson GS, Vorderman AL, et al. The health and educational status of adolescents with congenital rubella syndrome. Dev Med Child Neurol 1985; 27: 721–9. [DOI] [PubMed] [Google Scholar]
- 14.Al-Awaidy S, Griffiths UK, Nwar HM, et al. Costs of congenital rubella syndrome (CRS) in Oman: evidence based on long-term follow-up of 43 children. Vaccine 2006; 24: 6437–45. [DOI] [PubMed] [Google Scholar]
- 15.Roush SW, Roush Mph SW, McIntyre L, et al. Manual for the Surveillance of Vaccine-Preventable Diseases, 2013. CreateSpace, 2013. [Google Scholar]
- 16.Preblud SR, Serdula MK, Frank JA, Hinman AR. Current status of rubella in the United States, 1969–1979. J Infect Dis 1980; 142: 776–9. [DOI] [PubMed] [Google Scholar]
- 17.Munro ND, Sheppard S, Smithells RW, Holzel H, Jones G. Temporal relations between maternal rubella and congenital defects. Lancet 1987; 2: 201–4. [DOI] [PubMed] [Google Scholar]
- 18.Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982; 2: 781–4. [DOI] [PubMed] [Google Scholar]
- 19.Grant GB, Reef SE, Patel M, Knapp JK, Dabbagh A. Progress in rubella and congenital rubella syndrome control and elimination - worldwide, 2000–2016. MMWR Morb Mortal Wkly Rep 2017; 66: 1256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Töndury G, Smith DW. Fetal rubella pathology. J Pediatr 1966; 68; 867–79. [DOI] [PubMed] [Google Scholar]
- 21.Esterly JR, Oppenheimer EH. Pathological lesions due to congenital rubella. Arch Pathol 1969; 87: 380–8. [PubMed] [Google Scholar]
- 22.Chantler JK, Smyrnis L, Tai G. Selective infection of astrocytes in human glial cell cultures by rubella virus. Lab Invest 1995; 72: 334–40. [PubMed] [Google Scholar]
- 23.Lazar M, Perelygina L, Martines R, et al. Immunolocalization and distribution of rubella antigen in fatal congenital rubella syndrome. EBioMedicine 2016; 3: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Nguyen T, Pham VH, Abe K. Pathogenesis of congenital rubella virus infection in human fetuses: viral infection in the ciliary body could play an important role in cataractogenesis. EBioMedicine 2015; 2: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev 2000; 13: 571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birdsong M Generalized cytomegalic inclusion disease in newborn infants. J Am Med Assoc 1956; 162: 1305. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Cytomegalovirus (CMV) and Congenital CMV Infection. https://www.cdc.gov/cmv/clinical/congenital-cmv.html (accessed 28 May 2018).
- 28.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17: 355–63. [DOI] [PubMed] [Google Scholar]
- 29.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17: 253–76. [DOI] [PubMed] [Google Scholar]
- 30.Noyola DE, Demmler GJ, Nelson CT, et al. Early predictors of neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr 2001; 138: 325–31. [DOI] [PubMed] [Google Scholar]
- 31.Alarcon A, Martinez-Biarge M, Cabañas F, Hernanz A, Quero J, Garcia-Alix A. Clinical, biochemical, and neuroimaging findings predict long-term neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J Pediatr 2013; 163: 828–34.e1. [DOI] [PubMed] [Google Scholar]
- 32.Giannattasio A, Bruzzese D, Di Costanzo P, et al. Neuroimaging Profiles and Neurodevelopmental Outcome in Infants With Congenital Cytomegalovirus Infection. Pediatr Infect Dis J 2018; 37: 1028–33. [DOI] [PubMed] [Google Scholar]
- 33.Boppana SB, Fowler KB. Insight into long-term neurodevelopmental outcomes in asymptomatic congenital CMV infection. Pediatrics 2017; 140: e20172526. [DOI] [PubMed] [Google Scholar]
- 34.Kashden J, Frison S, Fowler K, Pass RF, Boll TJ. Intellectual assessment of children with asymptomatic congenital cytomegalovirus infection. J Dev Behav Pediatr 1998; 19: 254–9. [DOI] [PubMed] [Google Scholar]
- 35.Lopez AS, Lanzieri TM, Claussen AH, et al. Intelligence and academic achievement with asymptomatic congenital cytomegalovirus infection. Pediatrics 2017; 140: e20171517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gindes L, Teperberg-Oikawa M, Sherman D, Pardo J, Rahav G. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG 2008; 115: 830–5. [DOI] [PubMed] [Google Scholar]
- 37.Lipitz S, Hoffmann C, Feldman B, Tepperberg-Dikawa M, Schiff E, Weisz B. Value of prenatal ultrasound and magnetic resonance imaging in assessment of congenital primary cytomegalovirus infection. Ultrasound Obstet Gynecol 2010; 36: 709–17. [DOI] [PubMed] [Google Scholar]
- 38.Foulon I, Naessens A, Foulon W, Casteels A, Gordts F. Hearing loss in children with congenital cytomegalovirus infection in relation to the maternal trimester in which the maternal primary infection occurred. Pediatrics 2008; 122: e1123–e1127. [DOI] [PubMed] [Google Scholar]
- 39.Enders G, Daiminger A, Bäder U, Exler S, Enders M. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 2011; 52: 244–6. [DOI] [PubMed] [Google Scholar]
- 40.Colugnati FAB, Staras SAS, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis 2007; 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasari V, Smith C, Khanna R. Recent advances in designing an effective vaccine to prevent cytomegalovirus-associated clinical diseases. Expert Rev Vaccines 2013; 12: 661–76. [DOI] [PubMed] [Google Scholar]
- 42.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360: 1191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffiths P New vaccines and antiviral drugs for cytomegalovirus. J Clin Virol 2019; 116: 58–61. [DOI] [PubMed] [Google Scholar]
- 44.Kimberlin DW, Jester PM, Sánchez PJ, et al. Valganciclovir for Symptomatic Congenital Cytomegalovirus Disease. N Engl J Med 2015; 372: 933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Society for Maternal-Fetal Medicine (SMFM), Hughes BL, Gyamfi-Bannerman C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol 2016; 214: B5–B11. [DOI] [PubMed] [Google Scholar]
- 46.Schleiss MR. Congenital cytomegalovirus infection: molecular mechanisms mediating viral pathogenesis. Infect Disord Drug Targets 2011; 11: 449–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fowler KB, McCollister FP, Sabo DL, et al. A targeted approach for congenital cytomegalovirus screening within newborn hearing screening. Pediatrics 2017; 139: e20162128 10.1542/peds.2016-2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diener ML, Zick CD, McVicar SB, Boettger J, Park AH. Outcomes from a hearing-targeted cytomegalovirus screening program. Pediatrics 2017; 139: e20160789. [DOI] [PubMed] [Google Scholar]
- 49.Baud D, Gubler DJ, Schaub B, Lanteri MC, Musso D. An update on Zika virus infection. Lancet 2017; 390: 2099–109. [DOI] [PubMed] [Google Scholar]
- 50.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WTGH, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016; 65: 242–7. [DOI] [PubMed] [Google Scholar]
- 51.Moore CA, Staples JE, Dobyns WB, et al. Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 2017; 171: 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazin AN, Poretti A, Di Cavalcanti Souza Cruz D, et al. Computed tomographic findings in microcephaly associated with Zika virus. N Engl J Med 2016; 374: 193–5. [DOI] [PubMed] [Google Scholar]
- 53.Satterfield-Nash A, Kotzky K, Allen J, et al. Health and development at age 19–24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak - Brazil, 2017. MMWR Morb Mortal Wkly Rep 2017; 66: 1347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wheeler AC, Toth D, Ridenour T, et al. Developmental outcomes among young children with congenital Zika syndrome in Brazil. JAMA Netw Open 2020; 3: e204096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulkey SB, Arroyave-Wessel M, Peyton C, et al. Neurodevelopmental abnormalities in children with in utero zika virus exposure without congenital Zika syndrome. JAMA Pediatr 2020; 174: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cranston JS, Tiene SF, Nielsen-Saines K, et al. Association between antenatal exposure to Zika virus and anatomical and neurodevelopmental abnormalities in children. JAMA Netw Open 2020; 3: e209303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallet E, Flamand C, Rousset D, et al. ZIKA virus infection in pregnant women in French Guiana: More precarious-more at risk. PLoS Negl Trop Dis 2020; 14: e0008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016; 534: 267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian X, Nguyen HN, Song MM, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 2016; 165: 238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabriel E, Ramani A, Karow U, et al. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell 2017; 20: 397–406.e5. [DOI] [PubMed] [Google Scholar]
- 61.Lichinchi G, Zhao BS, Wu Y, et al. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 2016; 20: 666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabriel MM, Geyer L, McHugh C, et al. Impact of Universal Newborn Hearing Screening on cochlear implanted children in Ireland. Int J Pediatr Otorhinolaryngol 2020; 133: 109975. [DOI] [PubMed] [Google Scholar]
- 63.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev 2015; 11: CD005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological Features of Covid-19. N Engl J Med Published online June 12, 2020. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology 2020; 95: 77–84. [DOI] [PubMed] [Google Scholar]
- 66.Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020; 222: 521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zeng L, Xia S, Yuan W, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr 2020; 174: 722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]