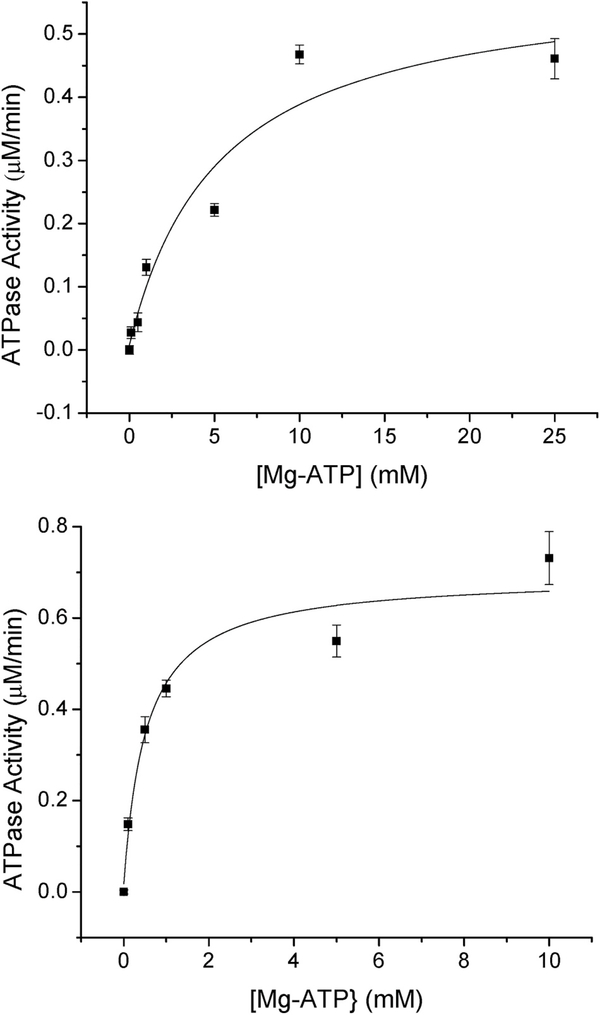
Fig. 3.
(Top) Mg-ATP dependence on ATP hydrolysis for native ABCB7. The dependence of Mg-ATP on ATPase activity for native ABCB7 was assayed using the EnzChek Phosphate Assay Kit. The KM for Mg-ATP was determined to be 5.3 ± 1.0 mM and the Vmax for ATPase activity was determined to be 0.58 ± 0.04 μM/min (bottom) Mg-ATP dependence on ATP hydrolysis for native ABCB7 in the presence of 10 μM [2Fe–2S](GS)4. The dependence of Mg-ATP on ATPase activity for native ABCB7 was assayed using the EnzChek Phosphate Assay Kit in the presence of 10 μM [2Fe–2S](GS)4. The KM for Mg-ATP was determined to be 0.54 ± 0.23 mM and the Vmax for ATPase activity was determined to be 0.68 ± 0.03 μM/min.