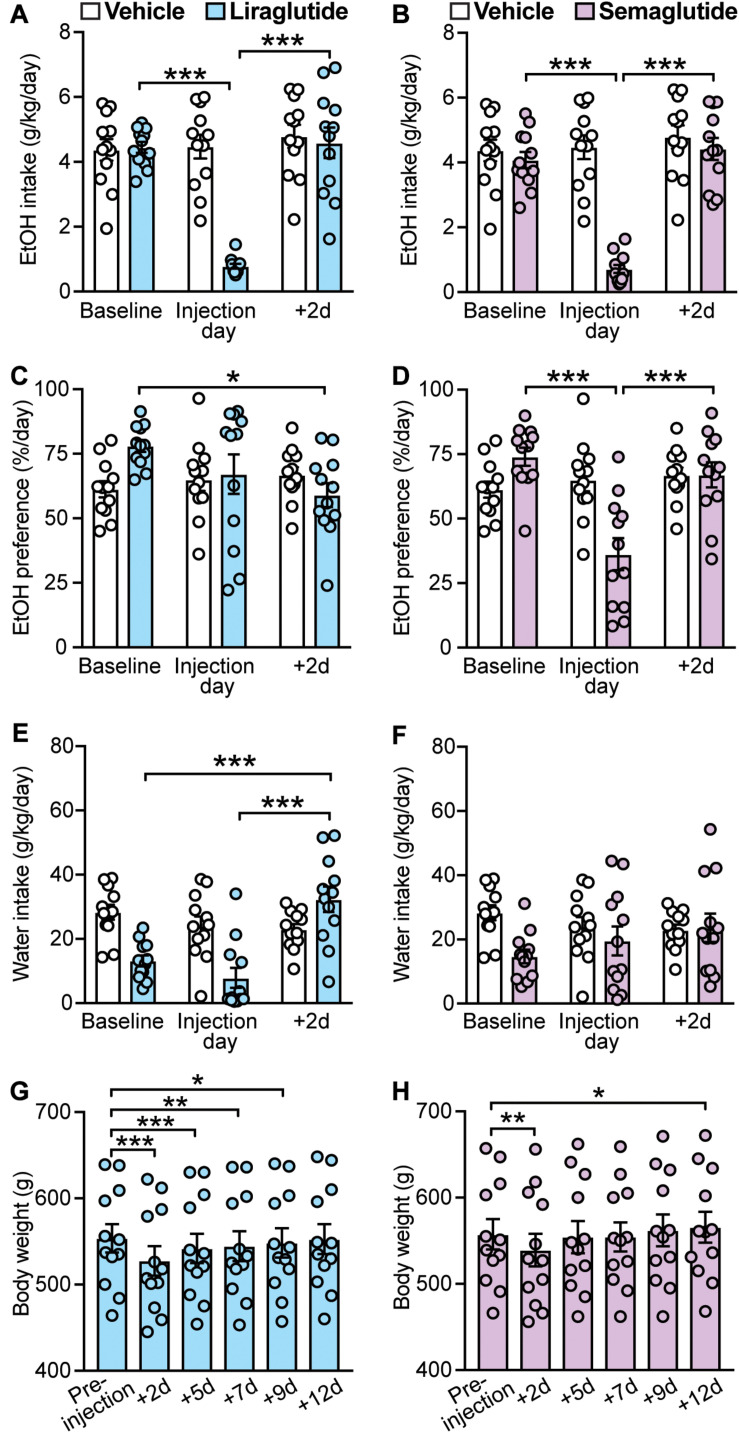
FIGURE 3.
Effects of GLP-1 analogs (liraglutide and semaglutide), compared to vehicle, on EtOH intake, preference, water intake, and body weight. Bars and circles represent the mean and individual data points, respectively. (A–F) Baseline represents the average of 3 presentations prior to vehicle/drug injection. (A) A significant treatment × time-point interaction effect on EtOH intake was found for liraglutide; under liraglutide, EtOH intake at the injection day was lower than baseline and 2-day post-injection (+ 2d). (B) A significant treatment × time-point interaction effect on EtOH intake was found for semaglutide; under semaglutide, EtOH intake at the injection day was lower than baseline and + 2d. (C) A significant treatment × time-point interaction effect on EtOH preference was found for liraglutide; under liraglutide, EtOH preference at + 2d was lower than baseline. (D) A significant treatment × time-point interaction effect on EtOH preference was found for semaglutide; under semaglutide, EtOH preference at the injection day was lower than baseline and + 2d. (E) A significant treatment × time-point interaction effect on water intake was found for liraglutide; under liraglutide, water intake at + 2d was higher than baseline and the injection day. (F) No significant treatment × time-point interaction effect on water intake was found for semaglutide. (G) Significant changes in body weight were observed under liraglutide; body weight at + 2d, + 5d, + 7d, and + 9d was lower than the body weight measured prior injection on injection day (pre-injection). (H) Significant changes in body weight were observed under semaglutide; body weight at + 2d was lower, and at + 12d was higher, than the body weight measured prior injection on injection day (pre-injection). *p < 0.05, **p < 0.01, ***p < 0.001.