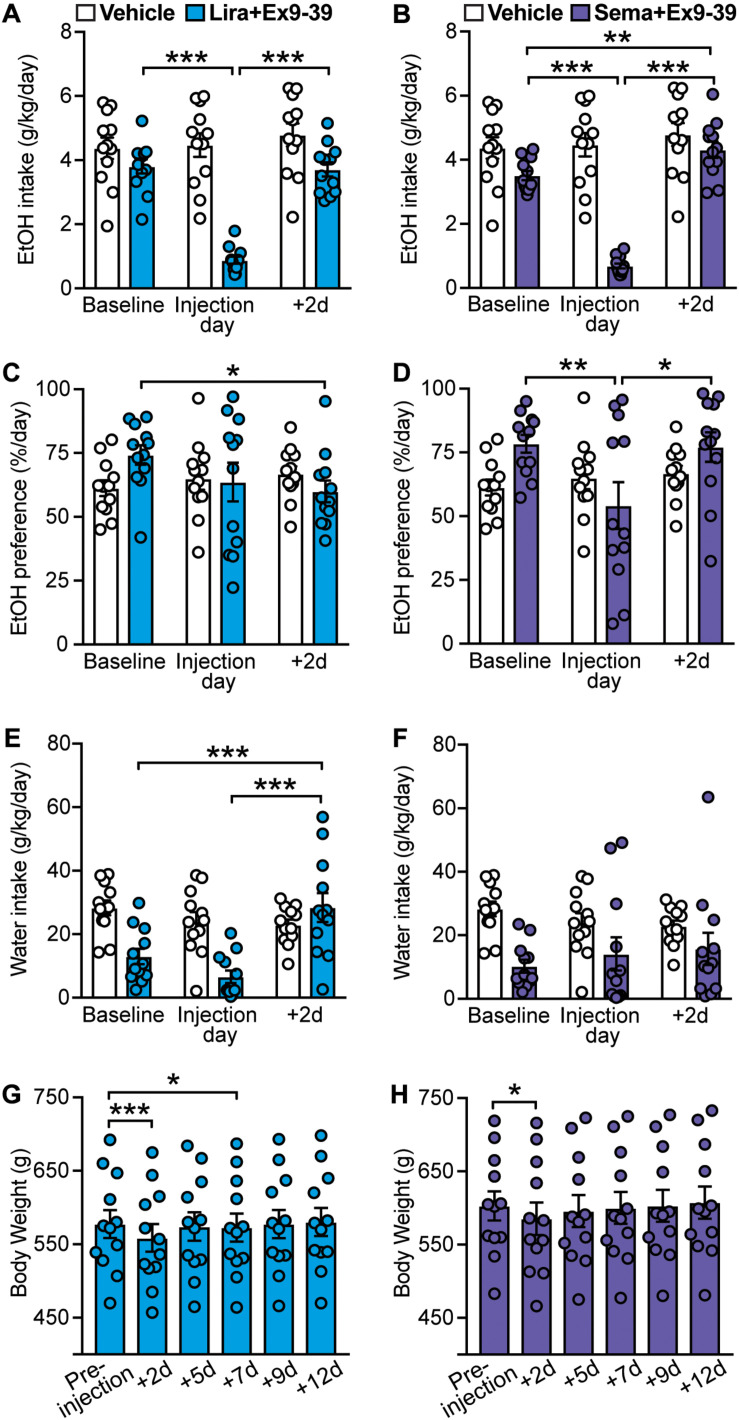
FIGURE 4.
Effects of GLP-1 analogs (liraglutide and semaglutide) co-administered with a GLP-1R antagonist (Ex9-39), compared to vehicle, on EtOH intake, preference, water intake, and body weight. Bars and circles represent the mean and individual data points, respectively. (A–F) Baseline represents the average of 3 presentations prior to vehicle/drug injection. (A) A significant treatment × time-point interaction effect on EtOH intake was found for liraglutide + Ex9-39; under liraglutide + Ex9-39, EtOH intake at the injection day was lower than baseline and 2-day post-injection (+ 2d). (B) A significant treatment × time-point interaction effect on EtOH intake was found for semaglutide + Ex9-39; under semaglutide + Ex9-39, EtOH intake at the injection day was lower than baseline and + 2d, and EtOH intake at + 2d was higher than baseline. (C) A significant treatment × time-point interaction effect on EtOH preference was found for liraglutide + Ex9-39; under liraglutide + Ex9-39, EtOH preference at + 2d was lower than baseline. (D) A significant treatment × time-point interaction effect on EtOH preference was found for semaglutide + Ex9-39; under semaglutide + Ex9-39, EtOH preference at the injection day was lower than baseline and + 2d. (E) A significant treatment × time-point interaction effect on water intake was found for liraglutide + Ex9-39; under liraglutide + Ex9-39, water intake at + 2d was higher than baseline and the injection day. (F) No significant treatment × time-point interaction effect on water intake was found for semaglutide + Ex9-39. (G) Significant changes in body weight were observed under liraglutide + Ex9-39; body weight at + 2d, and + 7d was lower than the body weight measured on injection day (pre-injection). (H) Significant changes in body weight were observed under semaglutide + Ex9-39; body weight at + 2d was lower than the body weight measured on injection day (pre-injection). *p < 0.05, **p < 0.01, ***p < 0.001.