Abstract
Background
In the last decade's robotic gastrectomy (RG) has increasingly widespread as a valid minimally invasive option for treatment of gastric cancer. In literature, evidence of its routine use is not yet well established. The aims of this study are to report our initial experience and to present possible advantages of our hybrid operative technique for subtotal gastrectomy.
Materials and methods
Retrospectively, we analyzed data from 41 patients (22 male and 19 female) who underwent robot-assisted laparoscopic subtotal gastrectomy (RALG) with D2 lymphadenectomy using the da Vinci XI robotic system. Inclusion criteria were gastric cancer in the middle or lower portion of the stomach amenable of radical subtotal gastrectomy without preoperative suspicion of positive lymph-nodes or other organs involving and distant metastasis. All the procedures were performed by attending surgeons.
Results
The mean operative time was 270 min with one case of conversion to open surgery. The mean age was 71.4 (IQR 68.2–76.8) with 43.9% of patients classified as ASA (American Society of Anesthesiologists) score ≥3. The median of lymph-nodes retrieved was 25 (IQR 19–35). No intra-operative complications occurred. Time to resume a soft diet was 5 days. Patients were hospitalized a median of 7 days. According to pathological AJCC-TNM, 21 patients were classified as advanced gastric cancer. Post-operative morbidity was recorded in 9 patients (21.9%) with major complications requiring surgical operation in 4 patients (9.8%). Elevated ASA score, fewer lymph-nodes retrieved and ICU recovery requirements were significant increased in patients with major complications.
Conclusion
The preliminary results demonstrated that robot-assisted laparoscopic subtotal gastrectomy is safe and feasible. In particular, we found that the da Vinci platform improves surgeon abilities to perform an adequate lymphadenectomy and digestive reconstruction. Further studies are necessary to better clarify the role of this high-cost technology in minimally invasive treatment of gastric cancer.
Keywords: Gastric cancer, Robot-assisted laparoscopic gastrectomy, Robotic surgery, Minimally invasive surgery, Distal gastrectomy, Case series
Highlights
-
•
Minimally invasive surgery for advanced gastric cancer is still a controversial issue.
-
•
Extended lymphadenectomy and digestive restoration represent the major drawback for minimally invasive approach.
-
•
Robotic surgical system overcomes some limitations of laparoscopic surgery.
-
•
RALG provides adequate oncological results in Western country without an extensive experience in laparoscopic gastrectomy.
1. Introduction
Gastric Cancer (GC) is the third leading cause of cancer-related deaths worldwide and the fifth most common cancer [1]. Standard gastrectomy with D2 lymphadenectomy remains the only curative option for resectable gastric cancer [2]. Since Kitano [3] first performed laparoscopy-assisted distal gastrectomy for early gastric cancer in 1994, minimally invasive approach for treating gastric cancer is being increasingly used. In the last decades several authors and trials [[4], [5], [6], [7], [8]] advocate laparoscopic gastrectomy (LG) as safe, feasible and oncological suitable in early-stage gastric cancer as it provides better outcomes compared to open gastrectomy in terms of post-operative recovery, reduced pain, faster recovery and more desirable cosmetic results. Despite these advantages laparoscopic D2 lymphadenectomy and digestive restoration are technically challenging with along learning curve [9,10], especially in advanced gastric cancer and obese patient. The da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) overcomes some of these disadvantages. Robot-assisted gastric surgery was first described in Italy [11] and in Japan [12]. After this initial experience several authors confirmed feasibility and safety of robot-assisted gastrectomy with reduction of surgery-related complications [[13], [14], [15], [16]]. Furthermore, many studies reported potential advantages of robotic gastrectomy compared to laparoscopic approach especially in European country with high BMI patients and more often with advanced cancer [17]. These advantages are particularly evident during D2 lymphadenectomy, which is one of the most important parameters to evaluate for oncological adequacy, and during complex digestive reconstructions such as the esophagojejunostomy. Moreover, fewer cases of robot-assisted laparoscopic gastrectomy are required to complete the learning curve as compared to laparoscopic gastrectomy [18]. The aims of present study are to present our hybrid operative technique and to report our preliminary experience with distal gastric cancer.
2. Material and Methods
Since 2016 the da Vinci XI Robotic System is being used in different surgical fields (general surgery, thoracic surgery, pediatric surgery, ORL surgery) at our hospital. In our department the da Vinci platform is mainly used to perform colorectal surgery and upper GI surgery. A single institution retrospective study of prospectively collected data of patients who underwent robot-assisted laparoscopic subtotal gastrectomy between September 2016 and March 2020 was performed. All the procedures were carried out by surgeons with great experience both in gastric surgery and advanced laparoscopic and robotic surgery (V.C. and M.B.). Patients underwent physical examination, upper GI endoscopy with biopsy, CT scan, PET-CT and in some case EUS for correctly cancer staging. The Eighth Edition of AJCC cancer manual staging was used for proper staging [19]. The study was registered at ResearchRegistry.com with an Identifying Number of 5876. Ethical committee approval was not necessary. This case series has been reported in line with the PROCESS Guideline [20].
2.1. Patient eligibility
Inclusion criteria were early gastric cancer (cT1) not indicated for endoscopic resection according to Japanese Gastric Cancer guidelines [2], tumor localized in the middle and lower portion of the stomach suitable for subtotal gastrectomy, resectable gastric cancer not involving other organs. Exclusion criteria were benign lesions of stomach, cancer localized in the esophagogastric junction or in the upper third of the stomach, multi-organs resection, pre-operative use of chemotherapy, metastatic disease and palliative resection. After adequate explanation of the procedure to all patients written informed consent was obtained.
2.2. Data collection
Data were collected in a data-base (Excel, Microsoft, USA): patient characteristic, short-term outcomes, pathological features, post-operative complications. Pathological outcomes were classified according to the Eighth Edition of AJCC cancer manual staging [19]. Pathological stage ≥ II underwent adjuvant chemotherapy. The frequency and severity of post-operative complications were determinated according to Clavien-Dindo (C-D) classification [21].
2.3. Statistical analysis
The statistical analysis was performed using IBM SPSS for statistics V.20. Continuous variables were expressed as median and interquartile range (IQR). Qualitative variables were compared using the Chi-square test or Fisher's exact test when appropriated. Continuous variables were compared using the Mann-Whitney U test. Survival rates were calculated using the Kaplan-Meyer method. A p-value <0,05 was considered statistically significant.
2.4. Operative technique
The patient is placed in supine position (Lloyds-Davies position) with the table tilted at 20° in reverse-Trendelenburg. The operator is located between the patient's legs, the assistant on the right side and the monitor is placed above the patient's head. The initial phase of the operation is performed laparoscopically. An umbilical 10 mm camera port is placed with open technique and pneumoperitoneum is maintained at 12 mmHg. Under direct visualization three additional ports are placed: 8 mm between the right midclavicular line and the midline, 8 mm between the left midclavicular line and the midline and 8 mm in the right upper quadrant at the anterior axillary line (Fig. 1). Exploration of the abdominal cavity is mandatory to reveal hepatic metastasis and peritoneal carcinosis. The gastro-colic ligament is then opened and right gastroepiploic vessels are divided using clips and ultrasound scalpel at the origin above the head of pancreas. Lesser sac is entered and pyloric artery is identified and divided. The posterior attachments of the stomach are divided and post-pyloric duodenal region is dissected for transection. Intraduodenal lymph-nodes are included in the specimen. An additional Airseal® (Conmed, NY, USA) 12 mm port is placed between umbilical port and left port. Duodenal transection is performed with a 60 mm stapler (blue load) through Airseal® port. A stitch is placed on the first digiunal loop in order to facilitate correct orientation during subsequent digestive restoration. After this phase umbilical camera port is replaced with 8 mm port (Fig. 2). The four-arm da Vinci XI system is positioned at the right side of the table for docking. D2 extended lymphadenectomy is carried out with monopolar hook (robotic arm R4) and bipolar forceps (robotic arm R2). Dissection begins along common hepatic artery (n.8) and hepato-duodenal ligament distally (n.12a). Celiac trunk (n.9) is dissected and the left gastric vessels are ligated (n.7) at their origin (Fig. 3). Lymph-nodes from the origin of splenic artery are dissected towards splenic hilum (n.11p). The lesser curvature of the stomach is skeletonized up to right crus of diaphragm (n.1). Stomach is divided along the greater curvature with 60 mm stapler (green load). Omentectomy is performed with ultrasound scalpel. All the specimens are placed into an endobag for removal through a supra-umbilical minilaparotomy incision. Billroth II antecolic manual anastomosis is fashioned at the posterior wall of the remnant stomach. The posterior serosal layer is sutured with interrupted stitches using Vicryl 3/0 (Ethicon Inc., USA). subsequently both the remnant stomach and the small intestine are opened. The internal layers are sutured with a running suture using autoblock suture (V-Loc™, Medtronic, MN, USA). Finally, interrupted stitches using Vicryl 3/0 (Ethicon Inc., USA) are used to suture the anterior serosal layer. A suction drain is placed close to duodenal stump and gastrojejunostomy. Usually the naso-gastric tube is not positioned.
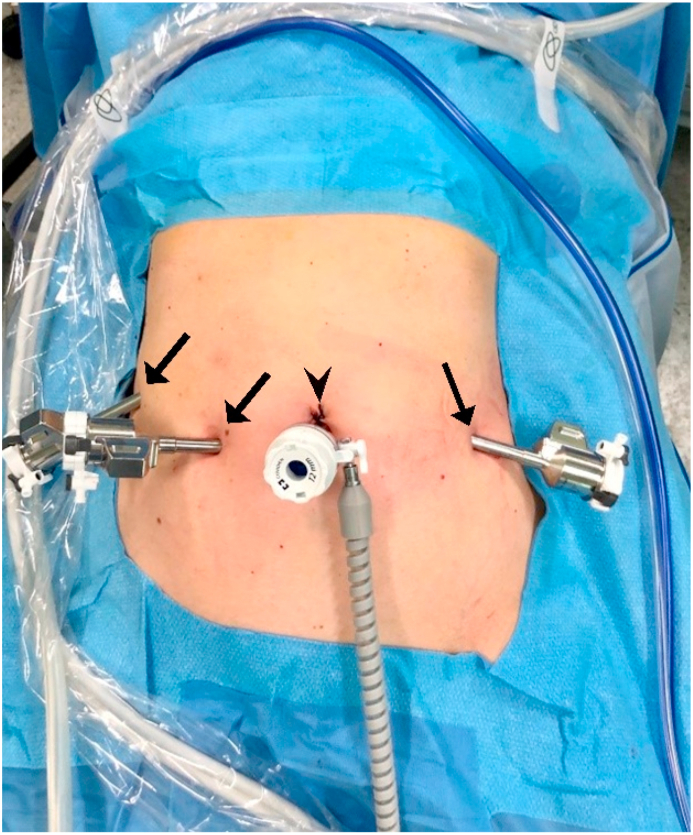
Fig. 1.
Port placement during laparoscopic phase. Black arrows indicate 8 mm port; black arrowhead indicates 10 mm camera port.
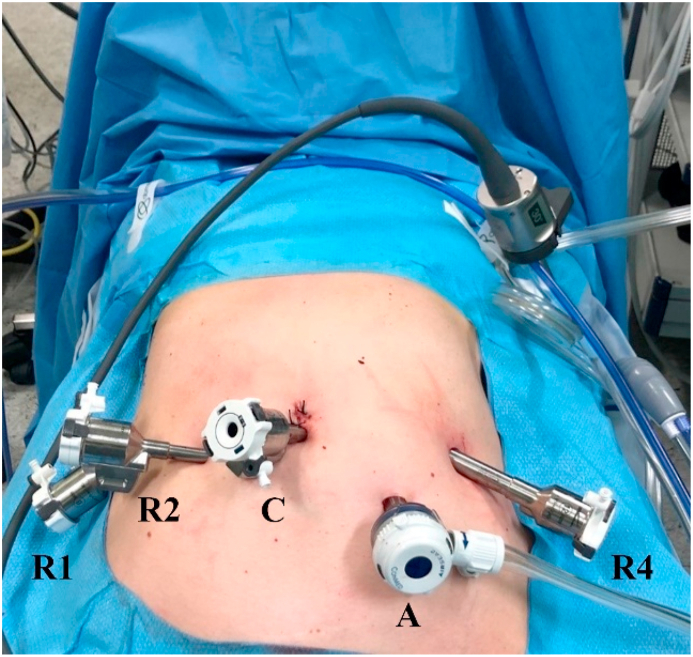
Fig. 2.
Port placement during robotic phase. R2 arm is equipped with bipolar forceps; R4 arm is equipped with monopolar hook; A is the assistant's port; C indicates camera port.
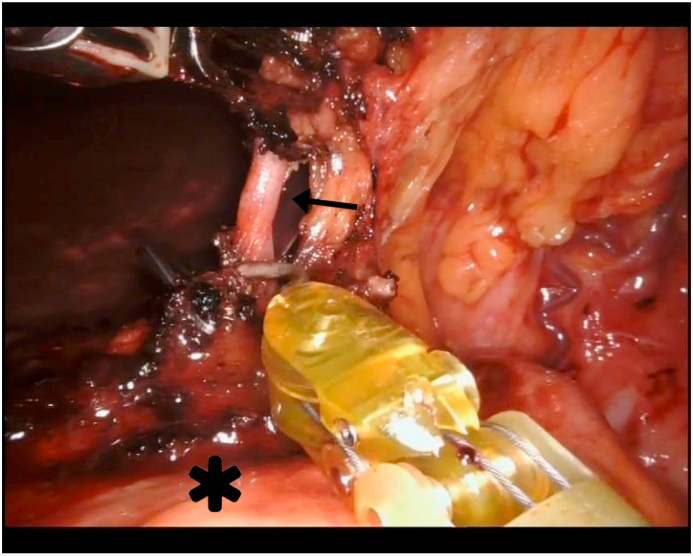
Fig. 3.
Robotic D2 lymphadenectomy. Black arrow is the origin of left gastric artery skeletonized; asterisk indicates pancreas.
3. Results
Forty-one consecutive patients underwent Robotic-assisted laparoscopic subtotal gastrectomy (RALG) for gastric cancer. The mean age was 71.4 (IQR 68.2–76.8) with 22 male and 19 female and the mean BMI was 26 (IQR 24.5–28); 43.9% of patients were classified ASA (American Society of Anesthesiologists) score ≥3 and 43.9% underwent prior abdominal surgery. In Table 1 patient's characteristics are summarized. The mean operative time was 270 min (IQR 252–300) with estimated blood loss (EBL) of 50 ml (IQR 50–100). No intraoperative complications occurred and no intraoperative blood transfusion was needed (see Table 2). One case was converted to open surgery (2.4%) because involvement of anterior surface of the pancreas with microscopic residual tumor on the specimen. The median of lymph-nodes retrieved was 25 (IQR 19–35). R0 resection was obtained in 40 patients (97.6%). According to pathological AJCC-TNM 51.2% of cases (21 patients) was considered as advanced gastric cancer (Stage ≥ IIA) and 61% was poorly differentiated (G3) tumor. Two patients (4.9%) were evaluated pathological Stage IV because of metastatic nodule of greater omentum on the specimen. Most tumors were located in the lower third of the stomach (80.5%). The median hospital stay was 7 days (IQR 6–9) and time to resume a soft diet was 5 days (see Table 3). Ten patients (24.4%) were admitted to ICU for a median of one day because of comorbidities. Post-operative morbidity was recorded in 21.9% (9 patients) and in 9.8% (4 patients) it was considered ≥ IIIa according to Clavien-Dindo (C-D) classification. Duodenal stump leak was observed in 2 cases and required conversion from BII to Roux en Y anastomosis. One patient had abdominal major bleeding on postoperative day (POD) 2 and one patient had iatrogenic ileal perforation on POD 1 both requiring re-intervention. All reoperations were performed with open surgery. The 30-days mortality was 2.4% and it occurred in the patient with preoperative ASA score of 4 that experienced duodenal stump leak. Clinical, surgical and pathological differences were analyzed between major complications group (C-D ≥ IIIa) and minor/none complications group (see Table 4). Elevated ASA score (p = 0.008), ICU recovery (p = 0.013), hospital stay (p = 0.014) and lymph-nodes retrieved (p = 0.001) were statistically different between two groups. In particular, age, BMI, pathological stage and operative time were not statistically different. The mean survival to recurrences was 31 months (standard error 0.125) with maximum follow-up of 34 months. During the first post-operative year, cancer recurrences were observed in 2 cases (4.9%) with advanced disease at time of surgery. One patient died of causes unrelated to gastric cancer: liver metastasis from breast cancer.
Table 1.
Patient features.
| Gender (M/F) | 22/19 |
|---|---|
| Age (years) | 71.4 (68.2–76.8)i |
| BMI (kg/m2) | 26 (24.5–28)i |
| Comorbidity - n (%) | |
| Systemic hypertension | 30 (73.2) |
| Diabetes mellitus | 7 (17.1) |
| Emphysema or COPD | 9 (21.9) |
| Coronary heart disease | 5 (12.2) |
| Chronic atrial fibrillation | 6 (14.6) |
| Chronic liver disease | 1 (2.4) |
| ASA Score – n (%) | |
| II | 23 (56.1) |
| III | 17 (41.5) |
| IV | 1 (2.4) |
| History of abdominal surgery – n (%) | 18 (43.9) |
Median and IQR.
Table 2.
Operative and pathological findings.
| Operative time (mins) | 270 (252–300)i |
| Estimated blood loss (ml) | 50 (50–100)i |
| Conversion - n (%) | 1 (2.4) |
| Intra-operative complications - n (%) | 0 (0) |
| Intra-operative blood transfusion - n (%) | 0 (0) |
| Pathological stage (AJCC-TNMii) - n (%) | |
| 0 | 1 (2.4) |
| IA | 15 (36.6) |
| IB | 4 (9.8) |
| IIA | 4 (9.8) |
| IIB | 3 (7.3) |
| IIIA | 9 (21.9) |
| IIIB | 3 (7.3) |
| IIIC | 0 (0) |
| IV | 2 (4.9) |
| Nodes retreived | 25 (19–35)i |
| Location - n (%) | |
| U (Upper) | 0 (0) |
| M (Middle) | 8 (19.5) |
| L (Lower) | 33 (80.5) |
| Grading - n (%) | |
| G1 | 4 (9.8) |
| G2 | 12 (29.2) |
| G3 | 25 (61.0) |
| Residual tumor - n (%) | |
| R0 | 40 (97.6) |
| R1 | 1 (2.4) |
| R2 | 0 (0) |
Median and IQR.
AJCC Cancer Manual Staging (8th Edition).
Table 3.
Short-term outcomes.
| ICU recovery - n (%) | 10 (24.4) |
| Time to diet (days) | |
| Liquid | 4 (3–5)i |
| Solid | 5 (5–6)i |
| Bowel function recovery (days) | 4 (3–5)i |
| Hospital stay (days) | 7 (6–9)i |
| Morbidity (overall complications) - n (%) | 9 (21.9) |
| Anastomotic bleeding | 0 (0) |
| Intra-abdominal bleeding | 1 (2.4) |
| Post-operative blood transfusion | 1 (2.4) |
| Duodenal stump leakage | 2 (4.9) |
| Anastomotic leakage | 0 (0) |
| Delayed gastric emptying | 2 (4.9) |
| Iatrogenic intestinal perforation | 1 (2.4) |
| Pancreatic fistula | 0 (0) |
| Heart failure | 0 (0) |
| Intra-abdominal abscess | 0 (0) |
| Intestinal obstruction | 0 (0) |
| Wound infection | 2 (4.9) |
| Clavien-Dindo grade ≥ IIIA - n (%) | 4 (9.8) |
| Reoperation - n (%) | 4 (9.8) |
| 30-days mortality - n (%) | 1 (2.4) |
Median and IQR.
Table 4.
Differences between post-operative complications.
| C-Di grade < IIIA/None | C-Di grade ≥ IIIA | p-value | |
|---|---|---|---|
| Gender - n (%) | 0.368 | ||
| Male | 19 (51.4) | 3 (75.0) | |
| Woman | 18 (48.6) | 1 (25.0) | |
| Age (years) | 71.4 (68.2–76.8)ii | 70.9 (60.5–77.6)ii | 0.719 |
| BMI (kg/m2) | 26 (25–28)ii | 24 (22–28)ii | 0.538 |
| Comorbidity - n (%) | |||
| Systemic hypertension | 27 (73.0) | 3 (75.0) | 0.931 |
| Diabetes mellitus | 6 (16.2) | 1 (25.0) | 0.657 |
| Emphysema or COPD | 7 (18.9) | 2 (50.0) | 0.154 |
| Coronary heart disease | 4 (10.8) | 1 (25.0) | 0.410 |
| Chronic atrial fibrillation | 5 (13.5) | 1 (25.0) | 0.537 |
| Chronic liver disease | 1 (2.7) | 0 (0) | 0.739 |
| ASA Score – n (%) | 0.008 | ||
| II | 21 (56.8) | 2(50.0) | |
| III | 16 (43.2) | 1(25.0) | |
| IV | 0 (0) | 1(25.0) | |
| History of abdominal surgery – n (%) | 15 (45.5) | 3 (75) | 0.187 |
| Pathological stage (AJCC-TNMiii) - n (%) | 0.820 | ||
| 0 | 1 (2.7) | 0 (0) | |
| IA | 13 (35.1) | 2 (50.0) | |
| IB | 4 (10.8) | 0 (0) | |
| IIA | 4 (10.8) | 0 (0) | |
| IIB | 3 (8.1) | 0 (0) | |
| IIIA | 8 (21.6) | 1 (25.0) | |
| IIIB | 2 (5.4) | 1 (25.0) | |
| IIIC | 0 (0) | 0 (0) | |
| IV | 2 (5.4) | 0 (0) | |
| Operative time (mins) | 270 (240–300)ii | 290 (266–342)ii | 0.382 |
| Estimated blood loss (ml) | 50 (50–100)ii | 50 (50–88)ii | 0.816 |
| Conversion - n (%) | 1 (2.7) | 0 (0) | 0.739 |
| Nodes retreived | 26 (20–38)ii | 15 (8–24)ii | 0.001 |
| ICU recovery - n (%) | 7 (18.9) | 3 (75.0) | 0.013 |
| Bowel function recovery (days) | 4 (3–5)ii | 5 (4–5)ii | 0.159 |
| Hospital stay (days) | 7 (6–9)ii | 11 (7–30)ii | 0.014 |
Qualitative variables were compared using Chi-Square Test or Fisher's exact test when appropriated.
Continuous variables were compared using Mann-Whitney U Test.
Clavien-Dindo classification.
Median and IQR.
AJCC Cancer Manual Staging (8th Edition).
4. Discussion
After Kitano [3] reported his initial experience of laparoscopy-assisted distal gastrectomy (LADG), minimally invasive surgery (MIS) for early gastric cancer is now widely accepted. Long-term results of KLASS01 [5] and JCOG0912 [7] trials, conducted in Korea and Japan, demonstrated the non-inferiority of LADG compared with open distal gastrectomy (ODG) for clinical stage I cancer in terms of overall survival (OS) and cancer-specific survival. These findings are supported in the recent JGCA guidelines [2] in which laparoscopic surgery can be considered as an option to treat early stage gastric cancer amenable of distal gastrectomy. Several authors [[22], [23], [24], [25], [26], [27]] sustain that laparoscopic gastrectomy with D2 lymphadenectomy can be performed with comparable short-term results to open approach for locally advanced gastric cancer (AGC), although long-term results from well-designed RCTs are awaited and current JGCA guidelines [2] recommend to perform MIS for AGC only in clinical trial setting. Certainly, laparoscopy-assisted gastric surgery provides better results in terms of reduction of operative blood loss, faster recovery, less pain, lower post-operative complications and shorter hospital stay compared to open gastrectomy [28,29]. Despite these results, laparoscopic D2 lymphadenectomy is a challenging and time-consuming procedure [10,30]. In particular, the dissection of the extragastric lymph-nodes station (n.7, 8, 9, 11p and 12a) with rigid and not articulated instruments requires longer mean operating time compared to OG groups with major fatigue for surgeons. Digestive reconstruction, especially after total gastrectomy, represents another major drawback in laparoscopy and not merely a shift from open end-to-side technique. It should be emphasized that most of high-quality studies originates from Eastern countries [10] conducted in large volume centers with a great experience in MIS of gastric cancer. Moreover, Western patients typically present with advanced lesions, higher proportion of proximal tumors and diffuse-type histology [31] together with high BMI and lower incidence of gastric cancer that render laparoscopic gastrectomy with D2 lymphadenectomy extremely demanding. Since these specific considerations and the importance to perform an adequate lymphadenectomy to reduce loco-regional recurrence and gastric cancer-related death [32], the robotic surgical system should be considerate an opportunity to overcome some of the major drawbacks of conventional laparoscopy. After an initial experience [11,12], several authors [[33], [34], [35], [36], [37], [38], [39]] demonstrated that robot-assisted laparoscopy gastrectomy is safe and feasible, with reduction in operative blood loss [34], longer operative time, lesser postoperative morbidity rate [16,40] and no difference in terms of harvested lymph-nodes when compared to laparoscopic and open approach [41]. The da Vinci system allows surgeon to perform a precise dissection of extra-gastric lymph-nodes, particularly in “difficult” stations such as no.7, 8, 9, 11p and 12a. In a large comparative retrospective study between 120 RAG vs 394 LAG, Junfeng [37] interestingly affirmed that robotic surgery is associated with a greater number of harvested lymph-nodes along tier 2. Also, Son [42] demonstrated that robotic dissection at the splenic hilum and artery results in a much larger amount of lymph-nodes retrieved compared to LAG. In our Department, the da Vinci surgical system was initially used in the treatment of colorectal cancer. After sufficient experience it was decided to improve our mini-invasive program with hybrid laparoscopic robot-assisted subtotal gastrectomy. In our opinion, exploratory laparoscopy is mandatory to verify feasibility of MIS and to avoid waste of cost consuming robotic materials. Indeed, Caruso [10] affirmed that the main criticism of this technology is the low ratio between the benefits compared to laparoscopic approach and the high cost of robotic procedure. Kim [35] compared 223 RG vs 211 LG and it was showed significantly longer operative time and higher total cost in robotic group (robotic = US$13,432 vs laparoscopic = US$8090; p < 0.001). For this reason, we suggest to perform the initial phase of the operation laparoscopically. Lysis of peritoneal adhesions is faster carried-out. Especially in advanced gastric cancer, laparoscopic division of gastro-colic ligament is important to rule out involvement of anterior surface of the pancreas and consequent conversion to open approach. Duodenal transection with mechanical stapler represents the last laparoscopic step before docking of the da Vinci platform. In our experience the robotic system enables surgeons to easily perform digestive restoration. Several studies [14,[43], [44], [45]] reported that extracorporeal anastomosis is safety performed through the same minilaparotomy used for specimen removal. Notably, in total gastrectomy with high BMI patients, it is necessary to perform a larger incision than a standard minilaparotomy for extracorporeal anastomosis with increasing risk due to lack of appropriate vision or excessive traction on the viscera [[46], [47], [48]]. In order to overcome these limitations, other authors proposed to carry out laparo-assisted intracorporeal anastomosis using stapler device with the Orvil [49] or the overlap technique [16,49,50]. Despite these solutions, using stapler device is associated with increasing cost of the procedure and with higher risk of leakage due to incomplete closure at the anastomotic level. Therefore, some authors [[51], [52], [53], [54], [55]] argued that the da Vinci system enable surgeons to perform a full robotic handsewn anastomosis with satisfying results, especially after total gastrectomy. Liu [53] demonstrated that robot-sewn digestive restoration, including esophagojejunal, gastroduodenal and gastrojejunal anastomoses, is feasible with fulfilling postoperative outcomes. Interestingly, Parisi [52] reported no postoperative complication after 55 consecutive patients underwent robotic total gastrectomy with double-loop reconstruction method (called Parisi technique). Although limited experience to subtotal gastrectomy without prior chemotherapy, in our opinion robotic manual anastomosis is safe and is associated with reduction in wound infection and intra-operative or post-operative anastomotic complications. In literature [18,55,56], it was demonstrated that robotic surgery requires a shorter learning curve as compared to conventional laparoscopy and that surgeons without extensive experience in laparoscopic gastrectomy can safety perform RADG with acceptable surgical outcomes. An [57] affirmed that surgeons with experience in open gastrectomy can easily achieve the stabilization of the duration of RADG after 25 cases. According to these findings, performing robotic total gastrectomy with handsewn intracorporeal anastomosis after an adequate learning curve with RADG represents a good option for patients. Nowadays, oncological adequacy of robotic gastrectomy is still a controversial issue. Despite RCTs on long-term outcomes are still lacking, many studies [37,[58], [59], [60], [61], [62]] reported that robotic gastrectomy for AGC is oncologically adequate when compared to LG or OG. Lately, Shin [63] demonstrated in a large propensity score-weighted analysis of 2084 consecutive patients underwent RAG vs LAG, no statistical difference in 5-years overall survival and 5-years recurrence-free survival after a mean follow up of 52 months. In a recent meta-analysis, Liao [64] compared 1009 RG vs 2401 LG with no significant differences in OS, DFS, RFS and recurrence rates between the two groups. It should be noted that all these studies are limited because retrospective, mainly derived from Eastern experience, with high heterogeneity groups and possible selection bias. As Coratti [17] affirmed, up to 30% of patients who underwent open gastrectomy are not able to receive adjuvant therapy because weakening of performance status and quality of life. Moreover, patients with AGC showed high rate of recurrence even during the first year after surgery [65]. It is our opinion that robot-assisted gastrectomy in conjunction with ERAS protocol [66] represent a lesser aggressive strategy to improve recovery after surgery and to increase the number of patients able to receive adjuvant chemotherapy. Especially in Western countries with more often AGC in high BMI patients, the faster recovery and better post-operative outcomes could justify the added cost of robotic surgery over open or laparoscopic surgery. The present study has several limitations. Firstly, this study is a retrospective case series. Despite this limitation, we found a reduction in hospital stay and post-operative morbidity. Secondly, this case series represents the initial phase of the learning curve for robot-assisted gastrectomy limited to gastric cancer suitable for subtotal gastrectomy. After achieving a sufficient experience, it is our intention to extend indications for total gastrectomy with handsewn intracorporeal anastomosis.
5. Conclusion
The purpose of this study was to demonstrated that robot-assisted subtotal gastrectomy is a safe and a valid alternative procedure when compared to standard open gastrectomy and conventional laparoscopy, even in Western countries. Our data regarding short-term outcomes and oncological adequacy appear to be satisfactory. Perioperative morbidity and 30 days mortality are satisfactory and the beginning of the learning curve such as the operative time needs to be taken in consideration. From an oncological point of view, we can only conclude that since no gastric stump resulted infiltrated by the cancer and patients received an adequate lymphadenectomy, our technique is adequate. We need to confirm these results with 5 years overall-survival as soon as the follow-up period is terminated. We found that hybrid laparoscopic and robotic technique is a reliable method to reduce operative time, emphasizing the beneficial effects of both approaches: lowering lost time related time-consuming procedures requiring different surgical fields of view and using robot dexterity for those highly precise and firm procedures represented by lymphadenectomy and anastomosis. In the future, well-designed RCTs, comparing robotic, laparoscopic and open gastrectomy regarding Western population, are necessary to clarify the benefits of this high-cost technology in terms of quality of life, overall survival, recurrence and disease-free survival.
Author contributions
Fabio Ambrosini: Conceptualization, Methodology, Investigation, Data Curation, Writing – Original draft preparation, Writing – Reviewing and Editing. Valerio Caracino: Conceptualization, Methodology, Writing – Original draft preparation, Supervision, Writing – Reviewing and Editing, Diletta Frazzini: Investigation, Data Curation, Pietro Coletta: Writing – Original draft preparation, Visualization, Edoardo Liberatore: Conceptualization, Investigation, Massimo Basti: Conceptualization, Investigation, Supervision. Giuseppe Di Martino: Formal analysis.
Guarantor
Fabio Ambrosini, Valerio Caracino.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Informed consent
Written informed consent was obtained from the patients for this study.
Ethical approval
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.12.026.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Japanese Gastric Cancer Association (JGCA) fifth ed. 2020. Japanese Gastric Cancer Treatment Guidelines 2018. Gastric Cancer, Online ahead of print. [DOI] [Google Scholar]
- 3.Kitano S., Iso Y., Moriyama M., Sugimachi K. Laparoscopy-assisted billroth I gastrectomy. Surg. Laparosc. Endosc. 1994;4:146–148. PMID: 8180768. [PubMed] [Google Scholar]
- 4.Kim W., Kim H.H., Han S.U., Kim M.C., Hyung W.J., Ryu S.W., Cho G.S., Kim C.Y., Yang H.K., Park D.J., Song K.Y., Lee S.I., Ryu S.Y., Lee J.H., Lee H.J. Korean laparo-endoscopic gastrointestinal surgery study (KLASS) group, decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01) Ann. Surg. 2016;263:28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.H., Han S.U., Kim M.C., Kim W., Lee H.J., Ryu S.W., Cho G.S., Kim C.Y., Yang H.K., Park D.J., Song K.Y., Lee S.I., Ryu S.Y., Lee J.H., Hyung W.J. Korean laparoendoscopic gastrointestinal surgery study (KLASS) group, effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–513. doi: 10.1001/jamaoncol.2018.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katai H., Mizusawa J., Katayama H., Takagi M., Yoshikawa T., Fukagawa T., Terashima M., Misawa K., Teshima S., Koeda K., Nunobe S., Fukushima N., Yasuda T., Asao Y., Fujiwara Y., Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20:699–708. doi: 10.1007/s10120-016-0646-9. [DOI] [PubMed] [Google Scholar]
- 7.Katai H., Mizusawa J., Katayama H., Morita S., Yamada T., Bando E., Ito S., Takagi M., Takagane A., Teshima S., Koeda K., Nunobe S., Yoshikawa T., Terashima M., Sasako M. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5:142–151. doi: 10.1016/S2468-1253(19)30332-2. [DOI] [PubMed] [Google Scholar]
- 8.Hyung W.J., Yang H.K., Han S.U., Lee Y.J., Park J.M., Kim J.J., Kwon O.K., Kong S.H., Kim H.I., Lee H.J., Kim W., Ryu S.W., Jin S.H., Oh S.J., Ryu K.W., Kim M.C., Ahn H.S., Park Y.K., Kim Y.H., Hwang S.H., Kim J.W., Cho G.S. A feasibility study of laparoscopic total gastrectomy for clinical stage I gastric cancer: a prospective multi-center phase II clinical trial, KLASS 03. Gastric Cancer. 2019;22:214–222. doi: 10.1007/s10120-018-0864-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim M.C., Jung G.J., Kim H.H. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J. Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caruso S., Patriti A., Roviello F., De Franco L., Franceschini F., Coratti A., Ceccarelli G. Laparoscopic and robot-assisted gastrectomy for gastric cancer: current considerations. World J. Gastroenterol. 2016;22:5694–5717. doi: 10.3748/wjg.v22.i25.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giulianotti P.C., Coratti A., Angelini M., Sbrana F., Cecconi S., Balestracci T., Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch. Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 12.Hashizume M., Sugimachi K. Robot-assisted gastric surgery. Surg. Clin. 2003;83:1429–1444. doi: 10.1016/S0039-6109(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C., Ellenhorn J., Hellan M., Pigazzi A. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg. Endosc. 2007;21:1662–1666. doi: 10.1007/s00464-007-9266-0. [DOI] [PubMed] [Google Scholar]
- 14.Song J., Oh S.J., Kang W.H., Hyung W.J., Choi S.H., Noh S.H. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann. Surg. 2009;249:927–932. doi: 10.1097/01.sla.0000351688.64999.73. [DOI] [PubMed] [Google Scholar]
- 15.Kang B.H., Xuan Y., Hur H., Ahn C.W., Cho Y.K., Han S.U. Comparison of surgical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: the learning curve of robotic surgery. J Gastric Cancer. 2012;12:156–163. doi: 10.5230/jgc.2012.12.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suda K., Man-I M., Ishida Y., Kawamura Y., Satoh S., Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg. Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 17.Coratti A., Annecchiarico M., Di Marino M., Gentile E., Coratti F., Giulianotti P.C. Robot-assisted gastrectomy for gastric cancer: current status and technical considerations. World J. Surg. 2013;37:2771–2781. doi: 10.1007/s00268-013-2100-z. [DOI] [PubMed] [Google Scholar]
- 18.Kim H.I., Park M.S., Song K.J., Woo Y., Hyung W.J. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur. J. Surg. Oncol. 2014;40:1346–1354. doi: 10.1016/j.ejso.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Amin M.B., Edge S., Greene F., Byrd D.R., Brookland R.K., Washington M.K., Gershenwald J.E., Compton C.C., Hess K.R., Sullivan D.C., Jessup J.M., Brierley J.D., Gaspar L.E., Schilsky R.L., Balch C.M. eighth ed. Springer International Publishing; 2017. American Joint Commission on Cancer, AJCC Cancer Staging Manual. [Google Scholar]
- 20.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A. N. O'Neill for the PROCESS group. The PROCESS 2020 guideline: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2020:60. doi: 10.1016/j.ijsu.2020.11.005. (article in press) [DOI] [PubMed] [Google Scholar]
- 21.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beyer K., Baukloh A.K., Kamphues C., Seeliger H., Heidecke C.D., Kreis M.E., Patrzyk M. Laparoscopic versus open gastrectomy for locally advanced gastric cancer: a systematic review and meta-analysis of randomized controlled studies. World J. Surg. Oncol. 2019;17 doi: 10.1186/s12957-019-1600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H.J., Hyung W.J., Yang H.K., Han S.U., Park Y.K., An J.Y., Kim W., Kim H.I., Kim H.H., Ryu S.W., Hur H., Kong S.H., Cho G.S., Kim J.J., Park D.J., Ryu K.W., Kim Y.W., Kim J.W., Lee J.H., Kim M.C. Korean laparo-endoscopic gastrointestinal surgery study (KLASS) group, short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with D2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT) Ann. Surg. 2019;270:983–991. doi: 10.1097/SLA.0000000000003217. [DOI] [PubMed] [Google Scholar]
- 24.Cai J., Wei D., Gao C.F., Zhang C.S., Zhang H., Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig. Surg. 2011;28:331–337. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 25.Park Y.K., Yoon H.M., Kim Y.W., Park J.Y., Ryu K.W., Lee Y.J., Jeong O., Yoon K.Y., Lee J.H., Lee S.E., Yu W., Jeong S.H., Kim T., Kim S., Nam B.H. COACT group, Laparoscopy-assisted versus open D2 distal gastrectomy for advanced gastric cancer: results from a randomized phase II multicenter clinical trial (COACT 1001) Ann. Surg. 2018;267:638–645. doi: 10.1097/SLA.0000000000002168. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y., Xu X., Zhao Y., Qian F., Tang B., Hao Y., Luo H., Chen J., Yu P. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg. Endosc. 2018;32:2427–2433. doi: 10.1007/s00464-017-5942-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z., Xing J., Cai J., Zhang Z., Li F., Zhang N., Wu J., Cui M., Liu Y., Chen L., Yang H., Zheng Z., Wang X., Gao C., Wang Z., Fan Q., Zhu Y., Ren S., Zhang C., Liu M., Ji J., Su X. Short-term surgical outcomes of laparoscopy-assisted versus open D2 distal gastrectomy for locally advanced gastric cancer in North China: a multicenter randomized controlled trial. Surg. Endosc. 2018;33:33–45. doi: 10.1007/s00464-018-6391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinuela E.F., Gonen M., Brennan M.F., Coit D.G., Strong V.E. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann. Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 29.Deng Y., Zhang Y., Guo T.K. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: a meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24:71–77. doi: 10.1016/j.suronc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Yakoub D., Athanasiou T., Tekkis P., Hanna G.B. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322–333. doi: 10.1016/j.suronc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Strong V.E., Song K.Y., Park C.H., Jacks L.M., Gonen M., Shah M., Coit D.G., Brennan M.F. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann. Surg. 2010;251:640–646. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 32.Songun I., Putter H., Kranenbarg E.M., Sasako M., van de Velde C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–449. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 33.Woo Y., Hyung W.J., Pak K.H., Inaba K., Obama K., Choi S.H., Noh S.H. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch. Surg. 2011;146:1086–1092. doi: 10.1001/archsurg.2011.114. [DOI] [PubMed] [Google Scholar]
- 34.Kim K.M., An J.Y., Kim H.I., Cheong J.H., Hyung W.J., Noh S.H. Major early complications following open, laparoscopic and robotic gastrectomy. Br. J. Surg. 2012;99(12):1681–1687. doi: 10.1002/bjs.8924. [DOI] [PubMed] [Google Scholar]
- 35.Kim H.I., Han S.U., Yang H.K., Kim Y.W., Lee H.J., Ryu K.W., Park J.M., An J.Y., Kim M.C., Park S., Song K.Y., Oh S.J., Kong S.H., Suh B.J., Yang D.H., Ha T.K., Kim Y.N., Hyung W.J. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann. Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 36.Park J.K., Kim Y.W., Ryu K.W., Eom B.W., Yoon H.M., Reim D. Emerging role of robot-assisted gastrectomy: analysis of consecutive 200 cases. J Gastric Cancer. 2013;13:255–262. doi: 10.5230/jgc.2013.13.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junfeng Z., Yan S., Bo T., Yingxue H., Dongzhu Z., Yongliang Z., Feng Q., Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg. Endosc. 2014;28:1779–1787. doi: 10.1007/s00464-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 38.Ye S., Shi J., Liu D.N., Jiang Q.G., Lei X., Tang B., He P.H., Zhu W.Q., Tang H.C., Li T.Y. Robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer based on propensity score matching: short-term outcomes at a high-capacity center. Sci. Rep. 2020;10:6502. doi: 10.1038/s41598-020-63616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan S., Tameron A., Murphy A., Hussain L., Dunki-Jacobs E., Lee D.Y. Robotic versus laparoscopic gastrectomy for gastric adenocarcinoma: propensity-matched analysis. Surg. Innovat. 2020;27:26–31. doi: 10.1177/1553350619868113. [DOI] [PubMed] [Google Scholar]
- 40.Uyama I., Suda K., Nakauchi M., Kinoshita T., Noshiro H., Takiguchi S., Ehara K., Obama K., Kuwabara S., Okabe H., Terashima M. Clinical advantages of robotic gastrectomy for clinical stage I/II gastric cancer: a multi-institutional prospective single-arm study. Gastric Cancer. 2019;22:377–385. doi: 10.1007/s10120-018-00906-8. [DOI] [PubMed] [Google Scholar]
- 41.Parisi A., Reim D., Borghi F., Nguyen N.T., Qi F., Coratti A., Cianchi F., Cesari M., Bazzocchi F., Alimoglu O., Gagnière J., Pernazza G., D'Imporzano S., Zhou Y.B., Azagra J.S., Facy O., Brower S.T., Jiang Z.W., Zang L., Isik A., Gemini A., Trastulli S., Novotny A., Marano A., Liu T., Annecchiarico M., Badii B., Arcuri G., Avanzolini A., Leblebici M., Pezet D., Cao S.G., Goergen M., Zhang S., Palazzini G., D'Andrea V., Desiderio J. Minimally invasive surgery for gastric cancer: a comparison between robotic, laparoscopic and open surgery. World J. Gastroenterol. 2017;23:2376–2384. doi: 10.3748/wjg.v23.i13.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son T., Lee J.H., Kim Y.M., Kim H.I., Noh S.H., Hyung W.J. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg. Endosc. 2014;28:2606–2615. doi: 10.1007/s00464-014-3511-0. [DOI] [PubMed] [Google Scholar]
- 43.Song K.Y., Kim S.N., Park C.H. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg. Endosc. 2008;22:655–659. doi: 10.1007/s00464-007-9431-5. [DOI] [PubMed] [Google Scholar]
- 44.Hyun M.H., Lee C.H., Kwon Y.J., Cho S.I., Jang Y.J., Kim D.H., Kim J.H., Park S.H., Mok Y.J., Park S.S. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann. Surg Oncol. 2013;20:1258–1265. doi: 10.1245/s10434-012-2679-6. [DOI] [PubMed] [Google Scholar]
- 45.Lee H.H., Hur H., Jung H., Jeon H.M., Park C.H., Song K.Y. Robot-assisted distal gastrectomy for gastric cancer: initial experience. Am. J. Surg. 2011;201:841–845. doi: 10.1016/j.amjsurg.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Lee J.H., Yom C.K., Han H.S. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg. Endosc. 2009;23:1759–1763. doi: 10.1007/s00464-008-0198-0. [DOI] [PubMed] [Google Scholar]
- 47.Noshiro H., Shimizu S., Nagai E., Ohuchida K., Tanaka M. Laparoscopy-assisted distal gastrectomy for early gastric cancer: is it beneficial for patients of heavier weight? Ann. Surg. 2003;238:680–685. doi: 10.1097/01.sla.0000094302.51616.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim K.H., Kim M.C., Jung G.J., Kim H.H. The impact of obesity on LADG for early gastric cancer. Gastric Cancer. 2006;9:303–307. doi: 10.1007/s10120-006-0395-2. [DOI] [PubMed] [Google Scholar]
- 49.Vasilescu C., Procopiuc L. Robotic surgery of locally advanced gastric cancer: a single-surgeon experience of 41 cases. Chirurgia. 2012;107:510–517. PMID: 23025119. [PubMed] [Google Scholar]
- 50.Zhang S., Khaliq J., Li D., Jiang X., Sun R., Li Y. Robotic total gastrectomy with π-shaped esophagojejunostomy using a linear stapler as a novel technique. World J. Surg. Oncol. 2018;16:238. doi: 10.1186/s12957-018-1542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parisi A., Ricci F., Trastulli S., Cirocchi R., Gemini A., Grassi V., Corsi A., Renzi C., De Santis F., Petrina A., Pironi D., D'Andrea V., Santoro A., Desiderio J. Robotic total gastrectomy with intracorporeal robot-sewn anastomosis: a novel approach adopting the double-loop reconstruction method. Medicine. 2015;94 doi: 10.1097/MD.0000000000001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parisi A., Ricci F., Gemini A., Trastulli S., Cirocchi R., Palazzini G., D'Andrea V., Desiderio J. New totally intracorporeal reconstructive approach after robotic total gastrectomy: technical details and short-term outcomes. World J. Gastroenterol. 2017;23:4293–4302. doi: 10.3748/wjg.v23.i23.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X.X., Jiang Z.W., Chen P., Zhao Y., Pan H.F., Li J.S. Full robot-assisted gastrectomy with intracorporeal robot-sewn anastomosis produces satisfying outcomes. World J. Gastroenterol. 2013;19:6427–6437. doi: 10.3748/wjg.v19.i38.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Z.W., Liu J., Wang G., Zhao K., Zhang S., Li N., Li J.S. Esophagojejunostomy reconstruction using a robot-sewing technique during totally robotic total gastrectomy for gastric cancer. Hepato-Gastroenterology. 2015;62:323–326. PMID: 25916057. [PubMed] [Google Scholar]
- 55.Kang B.H., Xuan Y., Hur H., Ahn C.W., Cho Y.K., Han S.U. Comparison of surgical outcomes between robotic and laparoscopic gastrectomy for gastric cancer: the learning curve of robotic surgery. J Gastric Cancer. 2012;12:156–163. doi: 10.5230/jgc.2012.12.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park S.S., Kim M.C., Park M.S., Hyung W.J. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg. Endosc. 2012;26:60–67. doi: 10.1007/s00464-011-1828-5. [DOI] [PubMed] [Google Scholar]
- 57.An J.Y., Kim S.M., Ahn S., Choi M.G., Lee J.H., Sohn T.S., Bae J.M., Kim S. Successful robotic gastrectomy does not require extensive laparoscopic experience. J Gastric Cancer. 2018;18:90–98. doi: 10.5230/jgc.2018.18.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pugliese R., Maggioni D., Sansonna F., Costanzi A., Ferrari G.C., Di Lernia S., Magistro C., De Martini P., Pugliese F. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg. Endosc. 2010;24:2594–2602. doi: 10.1007/s00464-010-1014-1. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y., Xi H., Qiao Z., Li J., Zhang K., Xie T., Shen W., Cui J., Wei B., Chen L. Comparison of robotic- and laparoscopic-assisted gastrectomy in advanced gastric cancer: updated short- and long-term results. Surg. Endosc. 2019;33:528–534. doi: 10.1007/s00464-018-6327-5. [DOI] [PubMed] [Google Scholar]
- 60.Obama K., Kim Y.M., Kang D.R., Son T., Kim H.I., Noh S.H., Hyung W.J. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. 2018;21:285–295. doi: 10.1007/s10120-017-0740-7. [DOI] [PubMed] [Google Scholar]
- 61.Li Z., Li J., Li B., Bai B., Liu Y., Lian B., Zhao Q. Robotic versus laparoscopic gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score-matched analysis. Canc. Manag. Res. 2018;10:705–714. doi: 10.2147/CMAR.S161007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coratti A., Fernandes E., Lombardi A., Di Marino M., Annecchiarico M., Felicioni L., Giulianotti P.C. Robot-assisted surgery for gastric carcinoma: five years follow-up and beyond: a single western center experience and long-term oncological outcomes. Eur. J. Surg. Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Shin H.J., Son S.Y., Wang B., Roh C.K., Hur H., Han S.U. Long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer: a propensity score-weighted analysis of 2084 consecutive patients. Ann. Surg. 2020 doi: 10.1097/SLA.0000000000003845. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Liao G., Zhao Z., Khan M., Yuan Y., Li X. Comparative analysis of robotic gastrectomy and laparoscopic gastrectomy for gastric cancer in terms of their long-term oncological outcomes: a meta-analysis of 3410 gastric cancer patients. World J. Surg. Oncol. 2019;17:86. doi: 10.1186/s12957-019-1628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ibáñez Aguirre F.J., Azagra J.S., Erro Azcárate M.L., Goergen M., Rico Selas P., Moreno Elola-Olaso A., Clemares de Lama M., de Simone P., Echenique Elizondo M.M. Laparoscopic gastrectomy for gastric adenocarcinoma. Long-term results. Rev. Esp. Enferm. Dig. 2006;98:491–500. doi: 10.4321/s1130-01082006000700002. [DOI] [PubMed] [Google Scholar]
- 66.Mortensen K., Nilsson M., Slim K., Schäfer M., Mariette C., Braga M., Carli F., Demartines N., Griffin S.M., Lassen K. Enhanced recovery after surgery (ERAS®) group, consensus guidelines for enhanced recovery after gastrectomy: enhanced recovery after surgery (ERAS®) society recommendations. Br. J. Surg. 2014;101:1209–1229. doi: 10.1002/bjs.9582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.