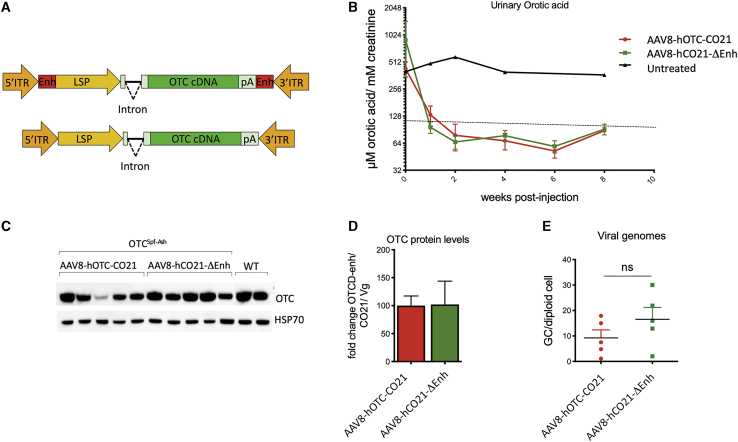
Figure 5.
Deletion of liver-specific enhancer sequences in the AAV vector backbone does not affect therapeutic efficacy
(A) The segments next to the AAV ITRs containing liver-specific transcription binding sites (Enh, indicated as red rectangles) were removed to generate the AAV8-hOTC-CO21ΔEnh vector (bottom). ITR, AAV2 inverted terminal repeat; intron, modified hemoglobin (HBB2) beta intron; LSP, liver-specific promoter (ApoE/hAAT, hybrid promoter containing ApoE enhancer and hAAT promoter); hOTC, WT or CO hOTC open reading frame (ORF); pA, hemoglobin beta (HBB) polyadenylation signal. (B) 3-month-old OTCSpf-Ash mice were i.v. injected with 5.0E11 vg/kg of AAV8-hOTC-CO21 or AAV8-hOTC-CO21ΔEnh (n = 5 per group). Animals were sacrificed 8 weeks after viral delivery. Urine samples were collected every 2 weeks post-injection and analyzed for orotic acid levels. Orotic acid values were standardized against creatinine levels. Dashed line delimits physiological level of orotic acid. (C) OTC protein levels were determined by WB analysis. (D) Densitometric quantification of the WB of (C), normalized by the vg copy number. Data are shown as mean ± SEM. (E) The vg particles were determined by quantitative real-time PCR, and the mean values of two independent determinations are indicated. Data are shown as mean ± SEM, and statistical analyses were performed by unpaired t test (∗p < 0.05).