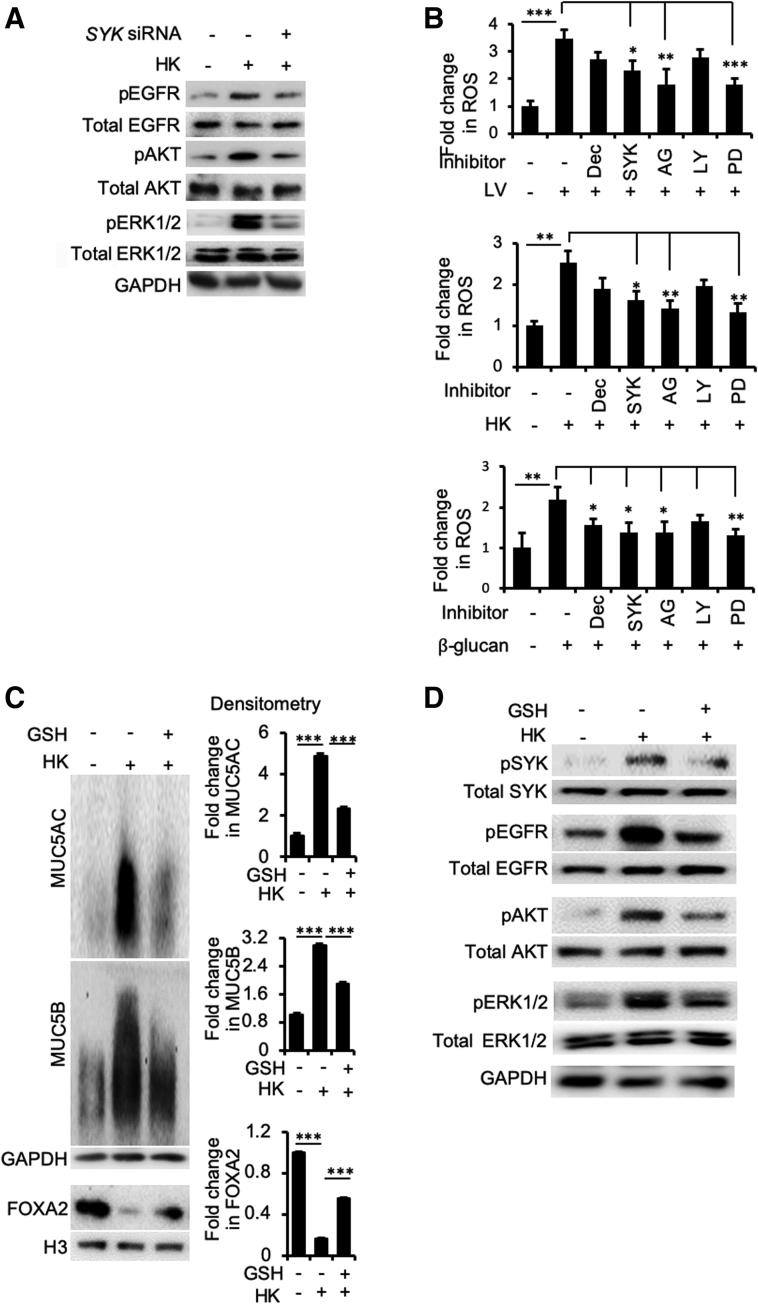
Figure 9.
Dectin-1 (Dec) and SYK act upstream of epidermal growth factor receptor (EGFR) signaling through up-regulation of reactive oxygen species (ROS). A: SYK acts upstream of EGFR-AKT/extracellular signal-regulated kinase (ERK) 1/2. Immortalized canine airway carcinoma (BACA) cells were pre-exposed to the siRNA against SYK before challenged with heat-killed (HK) Blastomyces dermatitidis strain SCB-2 (multiplicity of infection 5:1) for 24 hours. Control cells were exposed to the same volume of sterile phosphate-buffered saline. Membrane, cytoplasmic, and nuclear protein extracts were probed with regular or phospho-specific antibody against each protein. Total EGFR, AKT, and ERK1/2 were used as loading controls for their respective phosphorylated counterparts. H3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as additional loading controls. B: Live (LV) and HK B. dermatitidis and β-glucan induced intracellular levels of ROS that were attenuated by siRNA against CLEC7A (Dec) and SYK genes, or specific inhibitors against EGFR [AG1478 (AG)], AKT [LY294002 (LY)], and ERK1/2 [PD98059 (PD)] proteins. ROS levels were measured using the Oxiselect in vitro ROS/RNS assay kit. The experiments were performed independently three times in triplicate. C: Glutathione (GSH) reduces the expression of mucins by heat-killed B. dermatitidis. Additional replicates of Western blots can be found in Supplemental Figure S6. Expression levels of MUC5AC, MUC5B, and forkhead box protein A2 (FOXA2) were measured by using the ImageJ software version 1.52a. Densitometry data are shown. D: GSH attenuates SYK and EGFR signaling. The expression of each protein was determined by normal or phospho-specific antibodies. Total SYK, EGFR, Akt, ERK1/2, GAPDH, and H3 were used as loading controls. Data are expressed as means ± SEM from three experiments (B and C). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (t-test). pAKT, phosphorylated AKT; pEGFR, phosphorylated EGFR; pERK1/2, phosphorylated ERK1/2; pSYK, phosphorylated SYK.