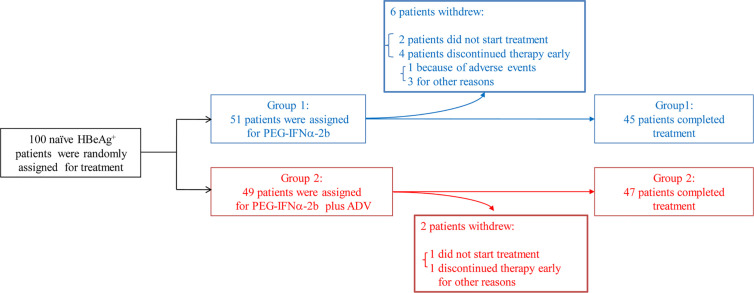
Figure 1.
Patients through the trial. One hundred patients that had been positive for HBeAg and hepatitis B surface antigen (HBsAg) for longer than 6 months and had elevated serum alanine transaminase (ALT) (>2 × ULN and <10 × ULN) and detectable baseline serum HBV DNA (>2 × 104 IU/ml) on at least two occasions have been involved into this clinical trial. Out of 100 HBeAg positive patients, 92 were completed the final Peg-IFNα-2b treatment. Randomized clinical trial were conducted including these 92 naive HBeAg-positive CHB patients. Patients were divided into two groups, one receiving Peg-IFNα-2b alone and one receiving Peg-IFNα-2b in combination with adefovir-dipivoxil in order to simulate patients undergoing treatment with nucleoside analog (NUC).