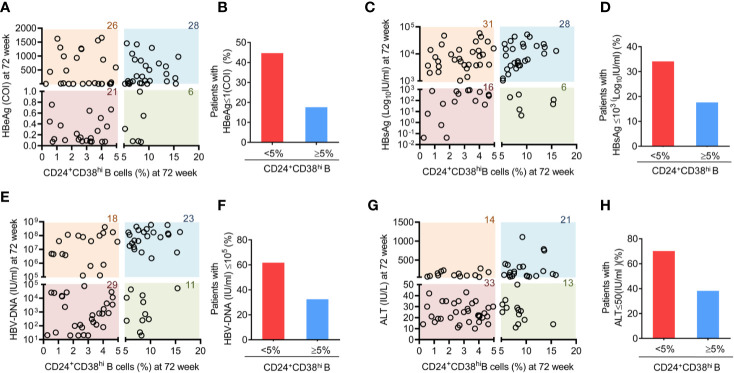
Figure 4.
Patients with fewer CD24+CD38hi B cells have an improved therapeutic effect. (A) Analysis between the percentage of CD24+CD38hi B cells in patients at 72 week and HBeAg expression at 72 weeks after the start of Peg-IFNα therapy. n = 81. (B) Percentage analysis of SRs among patients with fewer CD24+CD38hi B cells (<5%) group and more CD24+CD38hi B cells (≥5%) group. SR (seroconversion responder) was defined as the HBeAg seroconversion (COI < 1.0) at 72 weeks after the start of PEG-IFNα-2b therapy. (C, E, G) Analysis between the percentage of CD24+CD38hi B cells in patients at 72 week and HBsAg, HBVDNA and ALT expression at 72 weeks after the start of Peg-IFNα-2b therapy. n = 81. (D, F, H) Percentage analysis of patients with lower expression of HBsAg, HBVDNA and ALT expression at 72 weeks after the start of Peg-IFNα-2b therapy in fewer CD24+CD38hi B cells (<5%) group and more CD24+CD38hi B cells (≥5%) group. n = 81. Each circle in the Figure represents the data from a patient. The number marked on each color lump represent the sum number of patients.