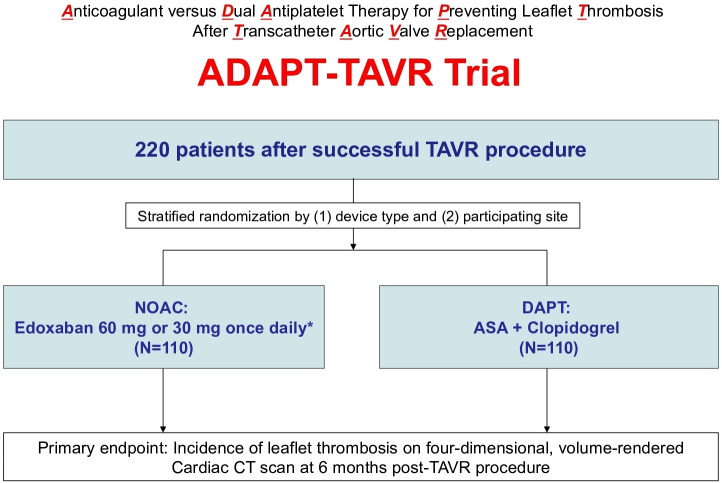
Figure 1.
Study flow diagram. Successful TAVR as defined in the ‘study population and methods’ section. *30 mg once daily if moderate or severe renal impairment (creatinine clearance 15 – 50 mL/min), low body weight ≤60kg or concomitant use of P-glycoprotein inhibitors (cyclosporin, dronedarone, erythromycin, ketoconazole). ASA, aspirin; DAPT, dual antiplatelet therapy; NOAC, non-vitamin K antagonist oral anticoagulant.