Summary
Background:
A prospective, pooled analysis of six randomized phase 3 trials was performed to evaluate disease-free survival regarding non-inferiority (NI) of three versus six months of adjuvant chemotherapy for stage III colon cancers. NI was not demonstrated. We now report the final overall survival results.
Methods:
Patients with age ≥18 years and performance score of 0-1 accrued from June 2007 to December 2015 across 12 countries in CALGB/SWOG80702, IDEA France, SCOT, ACHIEVE, TOSCA, and HORG trials (sample sizes: 708 to 3,983), who started any treatment (modified intent-to-treat) were included. Fluoropyrimidine and oxaliplatin with 5-FU/folinic acid (FOLFOX) or capecitabine (CAPOX) were delivered every 2 and 3 weeks, respectively. The primary endpoint was disease-free survival (time to relapse, secondary colorectal primary tumor, or death due to all causes) and that overall survival (time to death due to all causes) was the prespecified secondary endpoint. The overall survival NI margin was set to be HR=1·11. Pre-planned sub-group analyses included regimen and risk group. NI was to be declared if the one-sided false discovery rate adjusted (FDRadj) p-value was <0·025.
Findings:
With median follow-up of 72 months (IQR, 72·2-72·5), 2584 deaths among 12,835 patients were observed. Of all patients, 39·5% received CAPOX and 60·5% FOLFOX. Comparing three versus six months, the five-year overall survival rate was 82·4% and 82·8%, with an estimated hazard ratio (HR) of 1·02 (95% confidence interval [CI], 0·95-1·11; NI FDRadj p, 0·058); five-year overall survival rate was 82·1% versus 81·2% with an estimated HR of 0·96 (95% CI, 0·85-1·08; NI FDRadj p, 0·033) for CAPOX and 82·6% and 83·8%, estimated HR of 1·07 (95% CI, 0·97-1·18; NI FDRadj p, 0·34) for FOLFOX. Updated disease-free survival results confirmed previous findings. Adverse events data were not updated.
Conclusions:
While NI for overall survival could not be confirmed after multiplicity adjustment, the absolute 0·4% difference in five-year overall survival should be placed in clinical context. Overall survival results support the use of 3 months of adjuvant CAPOX for the vast majority of stage III colon cancer patients. This conclusion is strengthened by the substantial reduction of toxicities, inconveniencies, and cost associated with a shorter treatment duration.
Funding:
IDEA analyses were supported by a grant of U10 CA180882 from the National Cancer Institute.
Keywords: colon cancer, adjuvant therapy, oxaliplatin, 5-fluorouracil, capecitabine, neurotoxicity, overall survival
Introduction
Adjuvant chemotherapy for stage 3 colon cancer has been the standard of care for 30 years since the initial pivotal trial demonstrated a benefit in relapse-free and overall survival with postoperative treatment of 5-fluorouracil (5-FU) plus levamisole for duration of 12 months.1 Subsequently, in the mid-1990s, the Intergroup study 0089 established six months of 5-FU combined with folinic acid (FA) as standard of care in the adjuvant setting.2 In 2005, the ACCENT (Adjuvant Colon Cancer Endpoints) meta-analysis of adjuvant studies demonstrated that three-year disease-free survival was an excellent treatment effect predictor of five-year overall survival results and could be an appropriate primary endpoint for adjuvant studies in colon cancer.3 When oxaliplatin was introduced into the adjuvant treatment for patients with stage 3 colon cancer, a six-month duration of a combination with either infusional 5-FU/FA (FOLFOX) or capecitabine (CAPOX) became adjuvant standard of care due to a consistent, albeit moderate, disease-free survival benefit compared with 5-FU/FA alone.4–6 One of the main side effects of oxaliplatin-based adjuvant therapy is a cumulative sensory neurotoxicity, which can affect patient quality of life even long after the actual treatment is discontinued. Since the incidence and severity of neurotoxicity are correlated with the duration of oxaliplatin-based therapy, a critical clinical question was whether a shorter duration of therapy could achieve cancer outcomes as good as the standard six months of treatment, thereby reducing toxicity and health care utilization.
The International Duration Evaluation of Adjuvant therapy (IDEA) collaboration was established to determine, in form of a pre-planned, pooled analysis of six independently conducted trials, whether a three-month duration of oxaliplatin-based therapy was non-inferior to six months.7 In the overall population of 12,834 patients with stage 3 colon cancers, the IDEA-pooled analysis did not demonstrate non-inferiority (NI) regarding the primary endpoint, three-year disease-free survival, for three months versus six months of adjuvant FOLFOX/CAPOX. However, an unexpected interaction effect of the regimen used (FOLFOX versus CAPOX) was detected in a pre-planned analysis. In patients treated with CAPOX, a longer duration of therapy did not provide additional benefit with NI demonstrated (74.8% versus 75,9%; HR 0.95; 95% CI 0.85 to 1.06), whereas, six months of FOLFOX therapy was associated with moderate (73·6% versus 76%; HR 1.16; 95% CI, 1.06 to 1.26) but statistically improved three-year disease-free survival compared with three months. Of note, CAPOX versus FOLFOX was not randomly assigned in any of the IDEA trials; therefore, no direct outcomes comparison was possible between these two regimens.
Debates regarding the interpretation of the IDEA three-year disease-free survival results, as well as how to apply the results in clinical practice, have continued since the initial presentation in 2017.8 Questions have been raised regarding a potential difference in anti-tumor mechanisms of FOLFOX versus CAPOX, overall NI for a shorter adjuvant treatment was not proven for the whole population with yet very small differences in absolute three-year disease-free survival rates between duration groups and whether three-year disease-free survival can still reliably predict overall survival giving recent improvements in the treatment of metastatic disease after recurrence.7,9
Hence, the overall survival and updated disease-free survival comparisons are critical to provide definite inference regarding whether three months of adjuvant therapy is sufficient for stage 3 colon cancer patients. As all six IDEA studies have reached five years or longer follow-up, we now report the results for overall survival at five years together with the final five-year disease-free survival results.
Methods
Study Design and Participants
Individual patient data were pooled from six randomized phase 3 trials conducted in 12 countries (SCOT [NCT00749450; UK, Denmark, Spain, Australia, Sweden, New Zealand], TOSCA [NCT00646607; Italy], IDEA France [NCT00958737; France], Alliance/SWOG 80702 [NCT01150045; US, Canada], ACHIEVE [UMIN000008543; Japan], HORG [NCT01308086; Greece]).10–15 Stage III colon cancer patients (≥18 years old and performance score 0-1 per each study protocol) were eligible for this pooled analysis. Most of the studies adopted AJCC 6th for TNM staging classification, except ACHIEVE and IDEA France which used AJCC 7th staging. In both classification micrometastasis (size between 0·2 and 2 mm) are included since considered as stage III and isolated tumor cells in nodes non-included. The notion of tumor deposit only appeared in 2010 with AJCC 7th. All patients provided written, informed consent at enrollment in the respective trials.
Procedures
Patients in all trials were randomized to three versus six months of oxaliplatin-based treatment. Either 5-FU/FA (FOLFOX4 or modified FOLFOX6)4,16 or capecitabine (CAPOX)5,16, per discretion of the treating physicians, were allowed as the fluoropyrimidine backbone. Infusion LV 200(400) mg/m2, bolus 5-FU 400(400) mg/m2, infusion 5-FU 600(2400) mg/m2 and infusion oxaliplatin 85(85) mg/m2 were delivered every 2 weeks for 3 or 6 months for FOLFOX4(mFOLFOX6). Infusion oxaliplatin 130 mg/m2 and oral capecitabine 1000 mg/m2 twice-daily were delivered every 3 weeks for 3 or 6 months for CAPOX. Individual patient data of all trials were collected, and the analyses were performed in an independent statistical center at Mayo Clinic Rochester. The cut-off date for this analysis was January 20, 2020. No updated data on adverse events were transferred. The pooled analysis was approved by Mayo Clinic Investigational Review Board. Individual trials were approved through countries mechanisms at the time trials were conducted.
As previously stated,16 the primary endpoint was disease-free survival defined as time from date of randomization (enrollment) to the earliest date of relapse, secondary colorectal primary tumor, or death due to all causes. The underlying statistical assumptions for disease-free survival were detailed previously.16 The secondary endpoint was overall survival defined as time from date of randomization (enrollment) to the date of death due to all causes.
Statistical Analysis
All analyses were performed on an mITT population, which included stage 3 patients who were randomized and had received at least one treatment dose.
The NI margin for hypothesis testing regarding treatment effect for the overall survival endpoint was determined prior to performing the analyses. In order to balance between benefits (relief from neurotoxicity) and cost (loss of overall survival efficacy) due to a 50% reduction in oxaliplatin plus fluoropyrimidine exposure, the maximum acceptable loss of treatment efficacy was set to 1/2 of the OS gain obtained by adding oxaliplatin to 5FU/FA established in the MOSAIC trial (HR 0·80, 95% CI 0·66 to 0·96).17 Hence, the NI margin regarding overall survival endpoint was determined as HR=1/(0·8+(1-0·8)/2)=1·11. An estimated 2550 death events would provide approximately 75% power to declare overall survival NI of three months versus six months of treatment if there was no overall survival difference between two duration treatment groups at a one-sided type I error rate of 0·025, without multiplicity corrections.
In general, the Kaplan-Meier estimates and Cox models, stratified by study, were used to estimate the distributions of overall survival and disease-free survival and treatment effect in terms of HRs. The proportional hazard assumption for the stratified Cox model is examined using the scaled Schoenfield residuals.18 Q statistics and I2 values were used to assess the potential heterogeneity of study-specific HRs.
Following the initial report on three-year disease-free survival from IDEA,7 these subsequent analyses included secondary endpoint (overall survival) analyses, pre-planned subgroup analyses, updated primary endpoint (disease-free survival) analyses with additional data collections, and ad hoc analyses (Statistics analysis plan [SAP], in appendix p 9-23). To follow recently published New Guidelines for Statistical Reporting,19 point estimates of treatment-duration effects and standard error and/or two-sided 95% CIs were reported for both pre-planned and ad hoc analyses. Statistically significant testing and p-values were reported only for treatment duration comparisons considered for multiplicity adjustment (i.e., controlling type I error rate), by false discover rate method. These pre-specified comparisons in SAP included comparing disease-free survival (original reported and currently updated) and overall survival between three and six months of treatment, pooling all patients, within FOLFOX and CAPOX subgroups, and within high- and low-risk groups. When the observed one-sided false discovery rate adjusted (FDRadj) p-value was less than 0·025, the three months of treatment will be declared to be statistically non-inferior to six months of treatment. For superiority testing, two-sided FDRadj p-value was compared to significance cut-off of 0·05. Additional ad hoc analyses included subgroup analyses by T/N stage, number of lymph nodes examined, primary tumor location, historical grade, age, gender, and baseline performance score (PS). Analyses were performed using SAS software (version 9.4; SAS Institute Inc.)
Role of Funding Source
The IDEA collaboration and the pooled analyses efforts were supported by grant U10 CA180882 from the National Cancer Institute. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Authors of QS and JPM had access to the raw data.
The corresponding author had full access to all the data and had final responsibility to submit for publication.
Results
Across six trials, patients were accrued from June 2007 to December 2015. The overall median follow-up was 72.3 months (IQR, 72.2 to 72.5 months). The total sample size of the mITT population was updated to 12,835 from previously reported 12,834 due to additional information obtained for stage diagnosis and/or treatment data in the individual studies. Overall, no noticeable changes to patient characteristics distributions were previously reported.7 Table 1 lists summaries of updated T and N stage and risk group, primary tumor location, and updated median follow-up times for individual trials. As noted before, the percentage of patients with T4, N2, and high-risk tumors varied across studies. Importantly, the use of the chemotherapy regimens, CAPOX and FOLFOX, differed greatly between trials. At the time of data cut-off (January 2020), all studies had a median follow-up time beyond five years, ranging from 61·8 months (ACHIEVE) to 84·3 months (TOSCA).
Table 1:
Updated characteristics of the study population (modified intention-to-treat population)*
| TOSCA (N=2391) |
SCOT (N=3983) |
IDEA France (N=2010) |
CALGB/SWOG 80702 (N=2452) |
HORG (N=708) |
ACHIEVE (N=1291) |
Total (N=12835) |
|
|---|---|---|---|---|---|---|---|
| Tumor stage, no. (%) | |||||||
| T1 | 76 (3.2%) | 128 (3.2%) | 78 (3.9%) | 140 (5.8%) | 1 (0.1%) | 75 (5.8%) | 498 (3.9%) |
| T2 | 236 (10.0%) | 333 (8.4%) | 161 (8.0%) | 298 (12.3%) | 60 (8.5%) | 119 (9.2%) | 1207 (9.4%) |
| T3 | 1763 (74.5%) | 2347 (58.9%) | 1399 (69.6%) | 1623 (66.8%) | 549 (77.8%) | 734 (56.9%) | 8415 (65.8%) |
| T4 | 290 (12.3%) | 1174 (29.5%) | 372 (18.5%) | 368 (15.2%) | 96 (13.6%) | 363 (28.1%) | 2663 (20.8%) |
| Missing data | 24 | 0 | 0 | 23 | 2 | 0 | 49 |
| Nodal stage, no. (%) | |||||||
| N1 | 1745 (73.2%) | 2749 (69.0%) | 1501 (74.8%) | 1792 (73.8%) | 472 (67.2%) | 959 (74.3%) | 9218 (72.0%) |
| N2 | 640 (26.8%) | 1233 (31.0%) | 506 (25.2%) | 637 (26.2%) | 230 (32.8%) | 332 (25.7%) | 3578 (28.0%) |
| Missing data | 6 | 1 | 3 | 23 | 6 | 0 | 39 |
| Risk group, no. (%) | |||||||
| Low risk (T1, T2, or T3 N1) | 1545 (65.3%) | 2032 (51.0%) | 1245 (62.0%) | 1551 (63.9%) | 416 (59.1%) | 718 (55.6%) | 7507 (58.7%) |
| High risk (T4, N2, or both) | 820 (34.7%) | 1950 (49.0%) | 764 (38.0%) | 878 (36.1%) | 288 (40.9%) | 573 (44.4%) | 5273 (41.3%) |
| Missing data | 26 | 1 | 1 | 23 | 4 | 0 | 55 |
| Primary tumor sidedness, no. (%) | |||||||
| Proximal | 934 (40.8%) | NA | 750 (42.6%) | 1278 (53.7%) | 313 (44.5%) | 491 (38.5%) | 3766 (44.8%) |
| Distal | 1358 (59.2%) | NA | 1012 (57.4%) | 1102 (46.3%) | 390 (55.5%) | 784 (61.5%) | 4646 (55.2%) |
| Missing data | 99 | 3983 | 248 | 72 | 5 | 16 | 4423 |
| Chemotherapy regimen, no. (%) | |||||||
| CAPOX | 833 (34.8%) | 2649 (66.5%) | 201 (10.0%) | 0 (0.0%) | 412 (58.2%) | 969 (75.1%) | 5064 (39.5%) |
| FOLFOX | 1558 (65.2%)† | 1334 (33.5%) | 1809 (90.0%) | 2452 (100.0%) | 296 (41.8%) | 322 (24.9%) | 7771 (60.5%) |
| Median follow-up time, months (Q1, Q3) | 84.3 (83.0-85.8) |
75.2 (74.2-76.1) |
79.5 (78.4-81.0) |
66.2 (65.1-67.1) |
79.7 (74.7-81.7) |
61.8 (61.3-62.7) |
72.3 (72.2-72.5) |
Percentages may not total 100 because of rounding. TOSCA Three or Six Colon Adjuvant, SCOT Short Course Oncology Treatment, IDEA International Duration Evaluation of Adjuvant, CALGB/SWOG Cancer and Leukemia Group B/Southwest Oncology Group, ACHIEVE denotes Adjuvant Chemotherapy for Colon Cancer with High Evidence, and HORG Hellenic Oncology Research Group, Therapy.
Patients in this trial received FOLFOX4; those in the other trials received modified FOLFOX6.
Overall, no updated data to treatment compliance and adverse events were noted compared with the initial report.7 Treatment adherence, according to chemotherapy regimen and duration of therapy, was previously described.7 The percentage of patients who received all number of planned therapy was lower in the six-month therapy group (4,367 [68%] of 6,410 patients; median 24 weeks) than that in the three-month therapy group (5,681 [89%] of 6,356 patients; median 12 weeks). Neurotoxicity of grade 2 or higher per NCI-CTC, during active therapy and in the month after cessation of treatment, was substantially lower in the three-month therapy group (752 [16·0%] of 4,696 patients) than that in six-month therapy group (2,063 [44·5%] of 4,637 patients).
Firstly, we present the disease-free survival results of the pre-specified statistical hypothesis testing analyses with multiplicity adjustments. At the time of the January 2020 data lock, with nearly three years of additional follow-up, a total of 3781 disease-free survival events had occurred, which includes 518 added events from the original report.7 The original design required 3390 disease-free survival events for a statistical power of 90% for NI hypothesis testing at a one-sided significance level of 0·025. Among disease-free survival events, the percentage of death events without documentations of recurrence increased from 334 (10·2%) out of total 3263 events in February 2017 to 552 (14·6%) out of total 3781 events in January 2020.
In the overall mITT population, the updated HR for disease-free survival comparing three versus six months of treatment was 1·08, with a two-sided 95% CI of 1·02 to 1·15 (Figure 1). Comparing to six months of therapy, the disease-free survival rates in three months of therapy group at three years, four years, and five years decreased by 0·9% (95% CI of difference: −2·8% to 1·0%), 1·7% (95% CI of difference: −3·9% to 0·5%), and 1·7% (95% CI of difference: −4·2% to 0·8%), respectively. The statistical NI of three (versus six) months of adjuvant therapy remains unestablished for the whole study population (NI FDRadj p: 0·25, superiority FDRadj p for six months: 0·044). Neither violation to proportional hazards assumption (p=0·070), nor meaningful heterogeneities (I2=2·1%; Q p-value=0·40; appendix p 4) in HRs across individual studies was detected.
Figure 1:
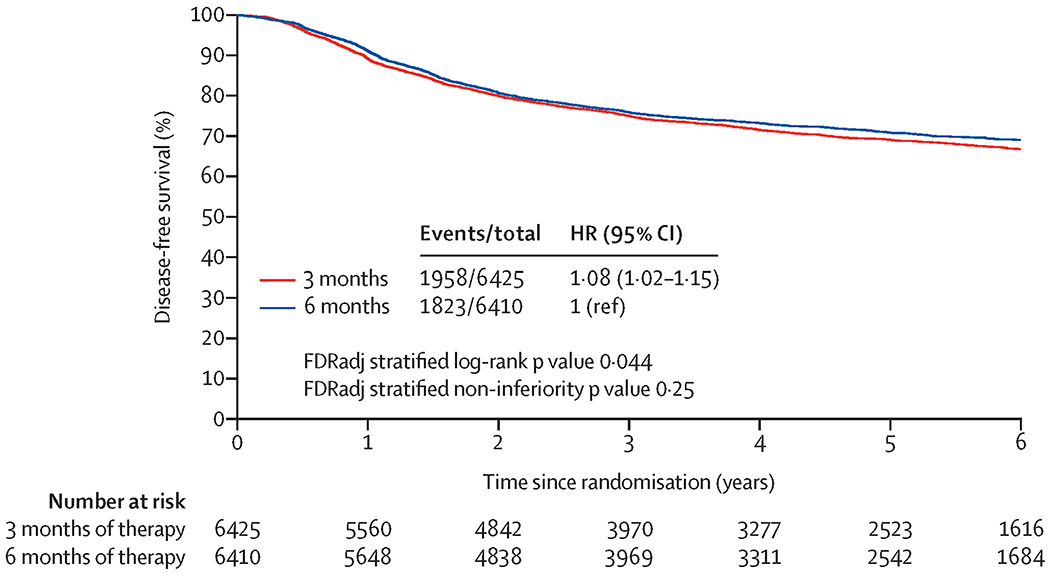
Updated disease-free survival with three months versus six months of adjuvant therapy in overall mITT Population
Comparing DFS between 3 vs. 6 months of therapy, the hazard ratio is 1.08 (95% confidence interval: 1.02 to 1.15). The statistical non-inferiority of 3 moths of therapy was not established in overall mITT population, since the observed one-sided false discover rate adjusted (FDRadj) non-inferiority p-value of 0.25 is larger than significance level of 0.025. The DFS of 6 months of therapy was significantly better than 3 months of therapy, since the observed two-sided FDRadj superiority p-value of 0.044 is smaller than the significance level of 0.05.
Table 2 displays the results for comparing disease-free survival in three versus six months of therapy groups in subgroups by regimen and risk groups. Overall, HR estimates were highly consistent with what was previously reported. With multiple comparison adjustment, the NI disease-free survival of three (versus six) months of therapy was barely missed the statistical significance for patients receiving CAPOX (HR: 0·98, 95% CI: 0·88 to 1·08, NI FDRadj p: 0·027). In contrast, disease-free survival of six months of therapy was statistically significantly improved over three months of therapy for patients receiving FOLFOX (HR: 1·16, 95% CI: 1·07 to 1·26, superiority FDRadj p: 0·0061). The P-value of testing interaction between regimen and duration was 0·011. There was no statistically significant evidence for NI of three (versus six) months of therapy for patients with low-risk (T1 - 3 and N1) tumors. However, six months of therapy showed superior disease-free survival compared to three months of therapy for patients deemed to have high-risk (T4 or N2) tumors, even after adjusting for multiplicity (Table 1). The P-value of testing interaction between risk group and duration was 0·24.
Table 2:
Treatment effects comparing disease free survival and overall survival between 3 months and 6 months of therapy with 6 months group as reference group
| Cohort | Disease Free Survival with 5 years of follow up | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) * | One-sided FDRadj p-value† for non-inferiority of 3 months therapy | Two-sided FDRadj p-value¥ for superiority of 6 months therapy | HR (95% CI) * | One-sided FDRadj p-value† for non-inferiority of 3 months therapy | Two-sided FDRadj p-value¥ for superiority of 6 months therapy | |
| Overall | 1.08 (1.02 to 1.15) | 0.25 | 0.044 | 1.02 (0.95-1.11) | 0.058 | 0.64 |
| CAPOX | 0.98 (0.88 to 1.08) | 0.027 | 0.67 | 0.96 (0.85-1.08) | 0.033 | 0.62 |
| FOLFOX | 1.16 (1.07 to 1.26) | 0.80 | 0.0061 | 1.07 (0.97-1.18) | 0.34 | 0.38 |
| Low Risk | 1.04 (0.94 to 1.15) | 0.16 | 0.58 | 0.95 (0.84-1.08) | 0.033 | 0.58 |
| High Risk | 1.13 (1.03 to 1.22) | 0.63 | 0.031 | 1.08 (0.98-1.19) | 0.39 | 0.29 |
HR denotes hazard ratio, CI confidence interval, FDRadj false discovery rate adjusted, mITT modified intention-to-treat
Two-sided 95% CI without adjustment of multicity;
If the observed one-sided FDRadj p-value is less than 0.025, then 3 months of therapy is declared statistically non-inferior to 6 months of therapy after adjusting for multicity.
If the observed two-sided FDRadj p-value is less than 0.05, then 6 months of therapy is declared statistically superior to 3 months of therapy after adjusting for multicity.
Secondly, we present the overall survival results of the pre-specified statistical hypothesis testing analyses with multiplicity adjustments. At the time of the January 2020 data lock, 2584 deaths had occurred. For the overall mITT population, the HR for comparing three versus six months of treatment was 1·02 with a two-sided 95% CI of 0·95 to 1·11 (Figure 2A). Thus, the upper bound of the two-sided 95% CI was equal to the pre-specified overall survival NI margin: HR of 1·11. The one-sided NI FRDadj p was 0·058. Accordingly, statistical NI of three (versus six) months of therapy was not established in the overall population at a stringent significance level (0·025) when adjusted for multiplicity testing. The five-year overall survival rates were 82·4% (95% CI: 81·4 to 83·3%) for the three-month arm compared with 82·8% (95% CI: 81·8 to 83·8%) for the six-month arm. The absolute difference of five-year overall survival rate between three versus six months of therapy was 0·4% (95% CI of difference: −2·1% to 1·3%; appendix p 1). Neither violation to PH assumption (p=0·52), nor meaningful heterogeneities (I2=0·0; Q p-value=0·58; appendix p 5) in HRs across individual studies were detected.
Figure 2:
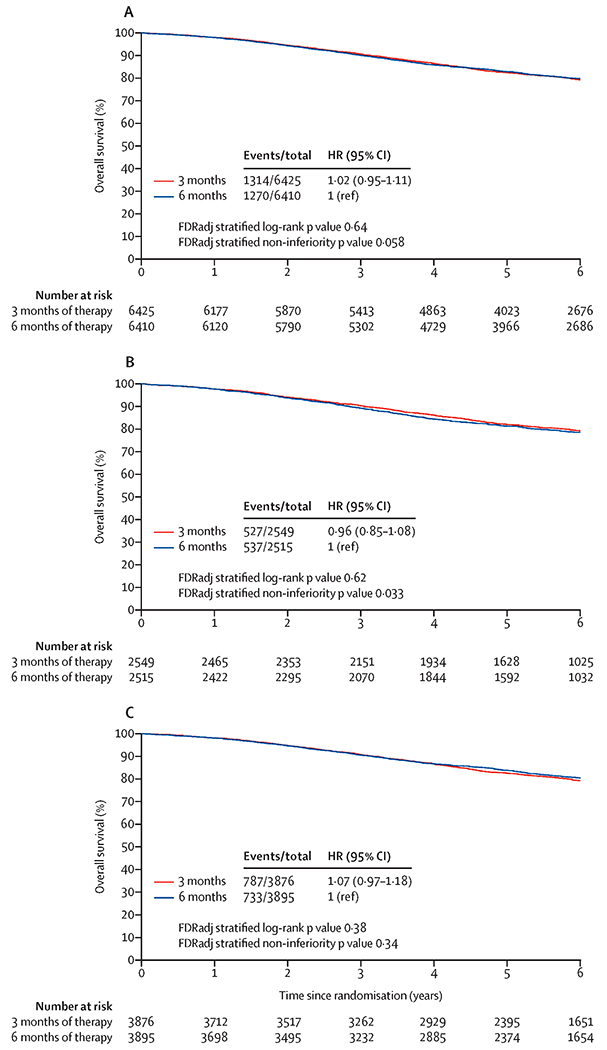
Overall survival with three months versus six months of adjuvant therapy; 2A: Overall mITT population; 2B: CAPOX; 2C: FOLFOX;
Among patients treated with CAPOX, similar to the findings for disease-free survival, NI of three (versus six) months of therapy for overall survival marginally missed statistical significance (one-sided FDRadj p: 0·033; HR 0·96 [95% CI: 0·85 to 1·08, without multiplicity adjustment]) and a five-year overall survival rate of 82·1% (95% CI: 80·5% to 83·6%) and 81·2% (95% CI: 79·7% to 82.9%) for three months and six months, respectively (difference: 0·9%, 95% CI: −1·8% to 3·6%, Figure 2B and appendix p 1). Among patients on FOLFOX, five-year overall survival rate of 82.6% (95% CI: 81·3% to 83·8%) and 83·8% (95% CI: 82·6% to 85·0%) for three months and six months, respectively, the HR for comparing three versus six months of treatment was 1·07, with a two-sided 95% CI of 0·97 to 1·18 (one-sided FDRadj p: 0·34, Figure 2C). The interaction p-value between regimen and duration was 0·20. These results stratified by regimen were strengthened by the consistency seen across studies. (see appendix p 2)
In the low-risk group, three months of oxaliplatin-based adjuvant therapy was marginally not non-inferior to a six-month duration (appendix p 3) with five-year overall survival rates of 89·6% (95% CI: 88·6% to 90·7%) and 88·9% (95% CI: 87·8% to 90·0%), respectively. For high-risk cancers, there was no statistically significant evidence of NI of three (versus six) months (appendix p 3) with five-year overall survival rates of 72·0% (95% CI: 70·3% to 73·8%) and 74·1% (95% CI: 72·4% to 75·9%), respectively. The interaction between duration and risk group was not statistically significant (pinteraction: 0·15).
Lastly, we present the results of the pre-planned subgroup analyses without multiplicity adjustments. Appendix p 6–7 displays the comparisons of outcomes between three versus six months of treatment within combination of regimens and risk groups. For patients receiving CAPOX, the numerically non-inferior disease-free survival and overall survival in three (versus six) months of therapy was observed in low-risk patients but not in high-risk patients. For patients receiving FOLFOX, the numerically inferior disease-free survival in three (versus six) months of therapy were consistently observed for both risk groups. However, for the overall survival endpoint, this numerically detrimental effect was less noticeable in the low-risk group.
Figure 3 displays the comparisons of outcomes, between three versus six months of treatment within other pre-specified subgroups. Overall, for disease-free survival endpoint, the point estimate of HR was consistently >1·0 with 95% CIs containing 1·0 for the majority of the subgroups. Furthermore, the treatment effect size comparing overall survival in patients assigned to three versus six months of therapy was consistently attenuated (i.e. HR point estimates were closer to 1·0 or became <1·0) compared to disease-free survival endpoint.
Figure 3:
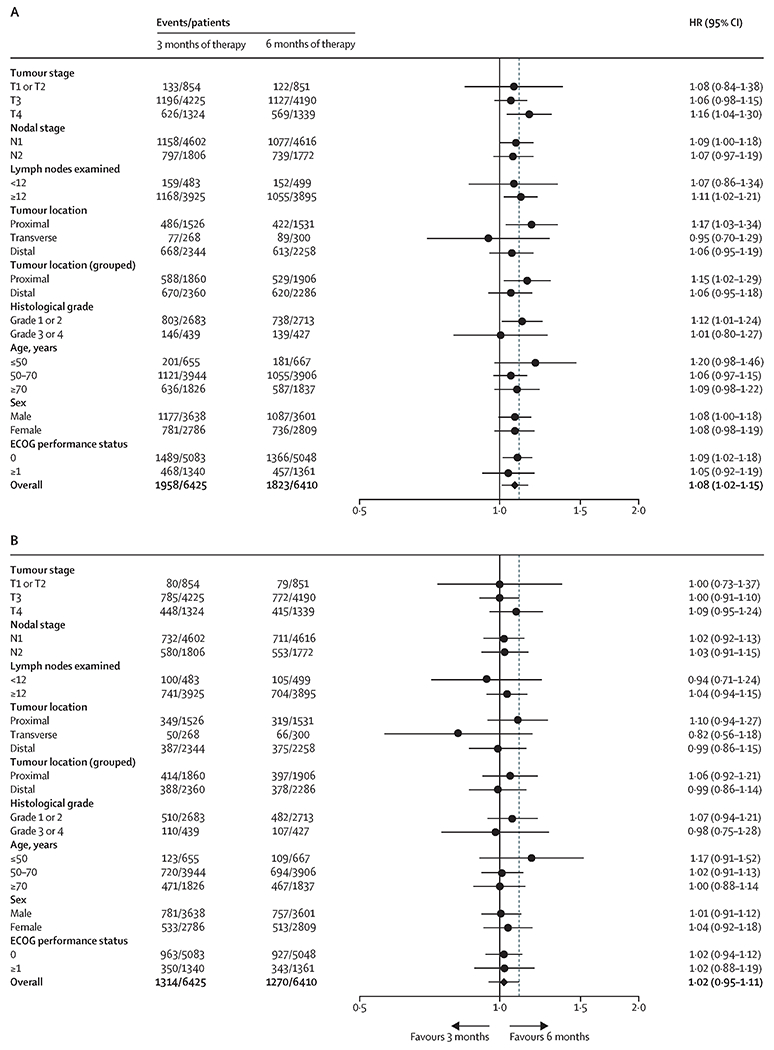
Comparing overall survival and disease-free survival between three months versus six months of adjuvant therapy in pre-defined subgroups; 3A: disease-free survival; 3B overall survival
Appendix p 8 displays survival curves of the low- and high-risk group regardless of regimen or duration for disease-free and overall survival. Comparing low- and high-risk patients, the disease-free rate was 83·5% (95% CI: 82·6% to 84·4%) versus 64·0% (95% CI: 62·7% to 65·3%) and 78·5% (95% CI: 77·5% to 79·5%) versus 57·7% (95% CI: 56·3% to 59·2%) at three and five years, respectively. The five-year overall survival rate was 89·3% and 73·1% in low- and high-risk group, respectively.
Discussion
Although the IDEA collaboration for stage III colon cancer did not meet prior statistical assumptions for NI in the overall population, the 0·4% difference in five-year overall survival has to be placed into clinical context, especially in light of the striking difference observed by regimen. Although no randomization between CAPOX and FOLFOX occurred, the outcomes analysis by regimen was a critical, pre-planned subgroup analysis since the launch of the IDEA collaboration. With further actualization of the primary endpoint of disease-free survival at five years, the statistical interaction effect between regimen and duration remains strong (pinteraction: 0·01). Consistently across analyses for three- and five-year disease-free survival, six months of FOLFOX shows significant better disease-free survival than three months of FOLFOX. In contrast, three months of CAPOX shows numerical better disease-free survival than six months of CAPOX, with marginally missing formal statistical significance for NI. Very similar data were seen for CAPOX regarding overall survival with numerically better results for three months (HR, 0·96; 95% CI, 0·85 to 1·08) versus six months but not for FOLFOX (HR, 1·07; 95% CI, 0·97 to 1·18). Therefore, any interpretation of the global IDEA results has to take the regimen effect into account.
As expected, shorter duration of adjuvant therapy was associated with significant reductions in adverse events, independent of the chemotherapy regimen.7 Most importantly, Grade 2 and higher neurotoxicity was markedly lower in the 3 months arm (FOLFOX 552 [16.8%] of 3,289 patients, CAPOX 200 [14.2%] of 1,407 patients) compared with 6 months (FOLFOX 1,440 [44.3%] of 3,252 patients, CAPOX 623 [45.0%] of 1,385). Additionally, diarrhea, neutropenia, thrombocytopenia, nausea, mucositis, fatigue, and hand-foot syndrome were also significantly reduced with shorter treatment duration.7 Shorter duration in this population of patients with an average age of 65 years old (up to 88 years old) avoid multiple clinic visits and the associated burden.
The statistical design of this large-scale prospective pooled analysis was with high standards. The methodology of our trial is much stronger compared with evidence emerging from adjuvant studies conducted in the 1990s, showing that a duration of adjuvant 5-FU and FA of 6 months is as effective as 12 months.2,20 The NI margins, the most critical design parameter for statistical hypothesis testing on both disease-free and overall survival endpoints, were determined based on rigorous statistical reasoning and clinical consensus among worldwide experts. Regarding the defined overall survival NI margin, our study acknowledged that due to the impact of subsequent treatment after recurrence (overall survival in this study for the whole population is 82.6% versus 76% in our hypothesis), the difference in overall survival between three and six months will be smaller compared with our prior assumptions for disease-free survival. The improved overall survival compared to the results achieved in MOSAIC (five-year overall survival rate of 76%) is important for the interpretation of IDEA since the MOSAIC results provided the statistical framework for our study. This may have contributed to the loss of power in IDEA for non-inferiority testing. We chose the overall survival NI margin, more narrow than the previous three-year disease-free survival NI margin. It should be noticed that the NI margins (translated to < 3% NI margins regarding absolute survival rates) chosen in IDEA design were much more stringent than previous NI surgical studies21,22 in early stage rectal cancer with NI 5 to 6% margins. To keep the risk of potential false-positive claims to a minimal level, the most stringent significance level, 0·025 for NI testing, with pre-planned multiplicity adjustment for secondary and subgroup analyses, were chosen. The unprecedented large sample size in colon cancers with fully matured follow-up ensured highly precise estimations of treatment effects and disease-free and survival rates at clinically relevant time points. Therefore, the clinical meaning of point estimates and confidence intervals should be the major considerations of data interpretations for oncologists and patients for shared decision-making on adjuvant therapy, rather than solely relying on “binary” statistical significance cut-off. We acknowledge that the results of our study probably present a challenging situation for data interpretation: the observed statistical p value crosses the very stringent significance cut-off to an extremely small degree. We are confident that precise estimations of treatment effects and survival rates, as presented here, can guide clinical interpretation and decision-making. The statistical method and strategy of our analysis are rigorous (advantage of the study) and stringent (making it more difficult to obtain “positive conclusions”).
Improvement of overall survival over time has multiple causes, in particular, improvement of outcomes after relapse due to progress in the management of metastatic disease.9 This assumption is in accordance with a recent meta-analysis of ACCENT,23 which confirmed 15 years after the initial publication by Sargent et al., that three-year disease-free survival is still an excellent predictor of five-year overall survival results and remains an appropriate primary endpoint for adjuvant trials in colon cancer.3
If CAPOX is selected as adjuvant therapy for stage III colon cancer, three months duration can be considered as the standard of care. The point estimate and 95% confidence intervals of the HR for overall survival and disease-free survival at five years for the whole group of patients treated with CAPOX fell within the pre-specified margins of NI. The same result was also observed in the low-risk group for CAPOX but not in the high-risk group. The HR of 1·03 and 1·05, concerning overall survival and disease-free survival, respectively, at five years in the high-risk CAPOX group, with only an absolute 1% non-significant difference in overall survival in favor of six months, are, in our opinion, a strong reason to consider three months of CAPOX as standard of care for all stage 3 colon cancer. If FOLFOX is selected as adjuvant therapy, six months with FOLFOX resulted in a higher rate in terms of disease-free survival and overall survival at five years, particularly in the high-risk subgroup. For high risk stage III cancers, emerging novel prognostic factors including immunoscore and/or circulating tumor DNA as marker for minimal residual disease, and potentially other advanced molecular prognostic tools may help in the future to better define the best duration of adjuvant therapy, especially for FOLFOX.24,25 For the low-risk subgroup, the outcomes detriment of three months of FOLFOX is minimal and should be discussed with the patient, again, outlining the risk of neurotoxicity.7,11,14
The administration of FOLFOX regimen is cumbersome since it requires the implantation of a venous access device and the use of an infusion pump. Capecitabine, an oral fluoropyrimidine, combined with oxaliplatin intravenously every three weeks is more convenient for the majority of patients, less expensive for the health care system in the vast majority of countries around the word, and does not need the placement of a venous access device for the majority of patients, especially if the duration of treatment is only three months, corresponding to only four IV administrations of oxaliplatin.26 Because no randomization between FOLFOX and CAPOX was performed in this study, it not possible to determine if three months of CAPOX is as good as six months of FOLFOX.7 In prior adjuvant trials, both regimens demonstrated efficacy in stage III colon cancer, with similar outcomes results. In particular, the Avastin® adjuVANT (AVANT) study randomized patients with stage 3 colon cancer to FOLFOX4, FOLFOX4 plus bevacizumab, and CAPOX plus bevacizumab.27 No difference in disease-free and overall survival was observed between the FOLFOX and CAPOX experimental arm, albeit in the presence of bevacizumab.
The lack of difference between three and six months of CAPOX could potentially be due to a reduced overall dose intensity in the six-month arm, with one hypothesis being that compliance and overall dose-intensity of six months of an oral regimen might attenuate over time. The documented dose intensity of capecitabine in the six-month arm, however, was not much different than the one of 5-FU, but the data were not based on rigorous diary-based documentation by individual patients.7 Alternative and potentially more relevant explanations relate to the specific dosing schedule of oxaliplatin (higher dose with 130 mg/m2 in CAPOX compared to 85 mg/m2 FOLFOX every three weeks instead of every two weeks) and the continuous mode of administration for the oral fluoropyrimidine (capecitabine twice daily for two weeks out of three in CAPOX, compared with 46-hour infusion of 5-FU every two weeks in FOLFOX), which might be a more optimized way to deliver cytotoxic chemotherapy in the adjuvant setting, with the intent to eradicate micrometastases.28,29 Although there were no major differences in baseline factors between patients treated with CAPOX vs. FOLFOX,7 further propensity analyses are planned to address potential selection biases with regard to the choice of treatment regimens in the adjuvant setting.
One important finding in our analysis for future adjuvant trial is a risk-based approach, with the fact that lower-risk groups (defined as T1-3 N1 disease) and higher-risk groups (T4 and/or N2) define two populations with a different prognosis, with 83·5% and 78·5 %, and 64·0% and 57·7% of disease-free survival at three and five years, respectively, and 89·3% and 73·1% of overall survival at five years, respectively, independent of the therapy duration. We suggest, in future studies use of these risk groups as stratification categories in randomized trials for all stage III colon cancer or to define specific populations to study subgroups of stage III disease.
This prospective, pre-planned pooled analysis of six concurrently conducted randomized phase 3 trials is the largest prospective, randomized analysis of adjuvant therapy of colon cancer to date. Despite the fact that, formally, the stringently pre-specified NI endpoints were not reached, the survival results support the use of three months of adjuvant CAPOX is the standard of adjuvant treatment for the most stage III colon cancer patients who are suitable for treatment with CAPOX. The final decision on treatment duration and regimen used for each individual will depend on a careful discussion between the clinician and patient, taking into account the risk of recurrence, comorbidity, patient’s wish, likely absolute difference in survival, and risk of long-term toxicity.
Supplementary Material
Research in context.
Evidence before this study
Six months of adjuvant oxaliplatin and fluoropyrimidine with either infusional 5-FU and folinic acid (FOLFOX) or capecitabine (CAPOX) has been the standard of care for patients with stage III colon cancer, as reflected in several international guidelines. In view of the cumulative peripheral neurotoxicity associated with oxaliplatin based on literature search in PubMed for reports published from Jan 01, 2000 to May 31, 2013 (in any language using the terms “adjuvant colon cancer”, “neurotoxicity”, and “oxaliplatin”), we investigated the question whether a shorter duration of treatment would reduce toxicity and health care utilization without sacrificing efficacy. In a stage III colon cancer population (N=12,834), the International Duration Evaluation of Adjuvant therapy (IDEA) collaboration initially did not demonstrate non-inferiority (NI) for three-year disease-free survival of three months versus six months of adjuvant FOLFOX/CAPOX. However, in a pre-planned analysis an unexpected interaction effect of the regimen used (FOLFOX versus CAPOX) was found. Six months of CAPOX did not provide statistically increased benefit compare to three months of t CAPOX. Debates regarding the interpretation of the IDEA three-year disease-free survival results, as well as how to apply the results in clinical practice, have continued since the initial presentation of the data in 2017.
Added value of this study
We now report the results for overall survival and updated disease-free survival at five years. This analysis confirms that three-year disease-free survival is an excellent predictor of overall survival at five years. While the overall survival results fail to reject the null hypothesis of non-inferiority in overall population, the absolute 0.4% difference in five-year overall survival has to be placed in clinical context, considering that non-inferiority was consistently observed for CAPOX but not for FOLFOX.
Implications of all the available evidence
Updated data from the IDEA collaboration with more than five years follow-up for outcomes confirm results obtained previously: three months of adjuvant chemotherapy with CAPOX is the standard of adjuvant treatment for most patients who are suitable for treatment with CAPOX. If the choice is to use FOLFOX, six months of treatment resulted in better disease-free survival, particularly in the clinical high-risk subgroup.
Acknowledgment
The IDEA collaboration and the pooled analyses efforts were in part supported by a grant of U10 CA180882 from the National Cancer Institute. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. None of authors are employed by NIH.
SCOT (NCT00749450) trial conduct was supported by a grant of EME 09/800/34 from the National Institute for Health Research, Efficacy and Mechanism Evaluation, the National Institute for Health Research, Health Technology Assessment, and a grant of C1348/A15960 from Cancer Research United Kingdom. TOSCA (NCT00646607) trial conduct was supported by a grant of FARM 5RWTWZ from L’Agenzia Italiana del Farmaco and AIRC IG21742–2018. IDEA France (NCT00958737) trial conduct was supported by Institut National du Cancer and a grant of PHRC2009 from Programme Hospitalier de Recherche Clinique en Cancérologie. Alliance/SWOG 80702 (NCT01150045) trial conduct was supported by grants U10CA180821, U10CA180835, UG1CA233163, U10CA180882, and U10CA180888 from the National Cancer Institute. ACHIEVE (UMIN000008543) trial conduct was supported by the Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC) and funded by Yakult Honsha Co, Ltd. HORG (NCT01308086) trial conduct was supported by the HORG Foundation.
We dedicate this work to the memory of Dr Daniel J. Sargent
Declaration of interest:
VG, AH, RL JPM, DN, ASh, ASo, VT, has nothing to disclose.
TA reports personal fees from Amgen, Astra Zeneca, Bristol-Myers-Squib, Chugai, Clovis Oncology, Gritstone Oncology, GlaxoSmithKline, Halliodx, Merck Sharp & Dohme Corp, Tesaro, Pierre Fabre, Roche/Vantana, Servier, Sanofi, Roche/Vantana, outside the submitted work.
IB reports personal fees and other from ASTRA-ZENECA, personal fees and other from BMS, personal fees and other from MSD, personal fees and other from NOVARTIS, personal fees and other from PFIZER, personal fees and other from ROCHE, personal fees and other from AMGEN, other from LILLY, personal fees and other from LEO, personal fees and other from PIERRE FABRE, personal fees from SANOFI, other from REGENERON, personal fees from SERVIER, personal fees and other from BOEHRINGER, outside the submitted work.
AG reports grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from Genentech/ Roche, grants and personal fees from Array/ Pfizer, grants and personal fees from Boston Biomedicals, grants from OBI Pharmaceuticals, grants from Merck, during the conduct of the study.
TI reports Honoraria from Eli Lilly, Bristol Myers Sqibb, Bayer, Amgen, Roche, Servier
TYo reports grants from Novartis Pharma K.K., grants from MSK K.K., grants from Sumitomo Dainippon Pharma Co., Ltd., grants from Chugai Pharmaceutical Co., Ltd., grants from Sanofi K.K., grants from Daiichi Sankyo Company, Limited, grants from PAREXEL International Inc., grants from ONO PHARMACEUTICAL CO., LTD., grants from Glaxo SmithKline K.K., outside the submitted work.
EO reports personal fees from Yakult Honsha, personal fees from Taiho Pharm, personal fees from Bayer Yakuhin, personal fees from Eli Lilly, personal fees from Chugai Pharm, personal fees from Ono Pharm, personal fees from Takeda Pharm, personal fees from Merck Biopharm, outside the submitted work.
TYa reports grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Bayer, grants from Takeda Pharmaceutical, grants from Taiho Pharmaceutical, outside the submitted work.
JM reports personal fees from Taiho Pharmaceutical, personal fees from Ignyta, personal fees from COTA Healthcare, outside the submitted work.
MS reports personal fees from SERVIER, personal fees from Merck, personal fees from Amgen, outside the submitted work.
QS reports other from Merk, other from Johnson & Johnson, other from Amgen, personal fees from Yiviva Inc, personal fees from Boehringer Ingelheim Pharmaceuticals, Inc , grants from Celgene, grants from Roche/Genentech, outside the submitted work.
IS reports grants from AMGEN, grants from ROCHE, grants and personal fees from MERCK SA, personal fees from SERVIER, grants and personal fees from SANOFI, personal fees from IPSEN, personal fees from MSD, outside the submitted work.
JT reports consulting/advisory role and or received honoraria from, Amgen, Haliodx, MSD Oncology, Astra-Zeneca, Pierre Fabre, Roche, Sanofi, Lilly, Servier and Merck KGAA and has received travel, accommodations, and expenses from Roche/Genentech, Celgene, Pierre Fabre, Servier and Merck KGAA.
VD reports personal fees from GERCOR, personal fees from INCYTE, personal fees from HALIODX, personal fees from CELLPROTHERA, outside the submitted work.
Appendix
Appendix p 1: Suppl. Table 1: Absolute difference (95% confidence interval) in 3, 4, and 5 years rates of outcomes between 3 months and 6 months of therapy with 6 months group as reference group
Appendix p 2: Suppl. Table 2: Comparing outcomes between 3 vs. 6 months of therapy by regimen in individual trials
Appendix p 3: Suppl. Figure 1: Overall survival with three months versus six months of adjuvant therapy by risk groups; Sup 1A: Low risk; Sup 1B: High risk
Appendix p 4-5: Suppl. Figure 2: Comparing outcomes between three versus six months of therapy in individual trials; Sup 2A: disease-free survival; Sup 2B: overall survival
Appendix p 6-7: Suppl. Figure 3: Comparing overall survival and disease-free survival between three months versus six months of adjuvant therapy in subgroups defined by combinations of regimen and risk groups; Sup 3A: disease-free survival; Sup 3B: overall survival
Appendix p 8: Suppl. Figure 4: Comparing overall survival and disease-free survival between risk groups; Sup 4A: disease-free survival; Sup 4B: overall survival
Appendix p 9-23: Statistical Analysis Plan
Footnotes
Data sharing statement: The data sharing of individual patient data from each participating trial will be subject to the policy and procedures of the institutions and groups who conducted the original study.
Contributor Information
Thierry André, Sorbonne Université and Hôpital Saint Antoine, 75012 Paris.
Jeffrey Meyerhardt, Dana-Farber Cancer Institute, Boston, MA, USA.
Timothy Iveson, University of Southampton, Southampton, United Kingdom.
Alberto Sobrero, Medical Oncology Unit, Ospedale San Martino, Genova, Italy.
Takayuki Yoshino, Department of Gastrointestinal Oncology, National Cancer Center Hospital East, Japan.
Ioannis Souglakos, Department of Medical Oncology, University Hospital of Heraklion, Faculty of Medicine, University of Crete, Greece.
Axel Grothey, West Cancer Center and Research Institute, OneOncology, Germantown, TN, USA.
Donna Niedzwiecki, Department of Biostatistics and Bioinformatics, Duke University, Durham, NC, USA.
Mark Saunders, Christie Hospital, Manchester, UK.
Roberto Labianca, Ospdale Papa Giovanni XXIII, Bergamo, Italy.
Takeharu Yamanaka, Yokohama City University School of Medicine Yokohama, Japan.
Ioannis Boukovinas, Bioclinic Thessaloniki Medical Oncology Unit, Thessaloniki, Greece.
Dewi Vernerey, Methodology and Quality of Life Unit, INSERM UMR 1098, 3 Boulevard Alexandre Fleming, 25030 Besançon, France.
Jeffrey Meyers, Department of Health Science Research, Mayo Clinic, Rochester, MN, USA.
Andrea Harkin, Operations Director, Cancer Research UK Glasgow Clinical Trials Unit; UK.
Valter Torri, IRRCS Mario Negri Institute for Pharmacological Research, Milano, Italy.
Eiji Oki, Department of Surgery and Science, Graduate School of Medical Science, Kyushu University, Japan.
Vassilis Georgoulias, University of Crete, Rethymno (UOC), Greece.
Julien Taieb, Université de Paris and Department of Gastroenterology and Gastrointestinal Oncology, Georges-Pompidou European Hospital, Paris, France.
Anthony Shields, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA.
Qian Shi, Department of Health Science Research, Mayo Clinic, Rochester, MN, USA.
References
- 1.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990; 322(6): 352–8. [DOI] [PubMed] [Google Scholar]
- 2.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005; 23(34): 8671–8. [DOI] [PubMed] [Google Scholar]
- 3.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005; 23(34): 8664–70. [DOI] [PubMed] [Google Scholar]
- 4.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350(23): 2343–51. [DOI] [PubMed] [Google Scholar]
- 5.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011; 29(11): 1465–71. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007; 25(16): 2198–204. [DOI] [PubMed] [Google Scholar]
- 7.Grothey A, Sobrero AF, Shields AF, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med 2018; 378(13): 1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobrero A, Grothey A, Iveson T, et al. The hard road to data interpretation: 3 or 6 months of adjuvant chemotherapy for patients with stage III colon cancer? Ann Oncol 2018; 29(5): 1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salem ME, Yin J, Goldberg RM, et al. Evaluation of the change of outcomes over a 10-year period in patients with stage III colon cancer: pooled analysis of 6501 patients treated with fluorouracil, leucovorin, and oxaliplatin in the ACCENT database. Ann Oncol 2020; 31(4): 480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andre T, Vernerey D, Mineur L, et al. Three Versus 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Patients With Stage III Colon Cancer: Disease-Free Survival Results From a Randomized, Open-Label, International Duration Evaluation of Adjuvant (IDEA) France, Phase III Trial. J Clin Oncol 2018; 36(15):1469–77. [DOI] [PubMed] [Google Scholar]
- 11.Iveson TJ, Kerr RS, Saunders MP, et al. 3 versus 6 months of adjuvant oxaliplatin-fluoropyrimidine combination therapy for colorectal cancer (SCOT): an international, randomised, phase 3, non-inferiority trial. Lancet Oncol 2018; 19(4): 562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobrero A, Lonardi S, Rosati G, et al. FOLFOX or CAPOX in Stage II to III Colon Cancer: Efficacy Results of the Italian Three or Six Colon Adjuvant Trial. J Clin Oncol 2018; 36(15): 1478–85. [DOI] [PubMed] [Google Scholar]
- 13.Souglakos J, Boukovinas I, Kakolyris S, et al. Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann Oncol 2019; 30(8): 1304–10. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino T, Yamanaka T, Oki E, et al. Efficacy and Long-term Peripheral Sensory Neuropathy of 3 vs 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Colon Cancer: The ACHIEVE Phase 3 Randomized Clinical Trial. JAMA Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerhardt JA, Shi Q, Fuchs CS, et al. Celecoxib in addition to standard adjuvant therapy with 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX) in stage III colon cancer: Results from CALGB/SWOG 80702. Journal of Clinical Oncology 2020; 38(15_suppl): 4003-. [Google Scholar]
- 16.Andre T, Iveson T, Labianca R, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective Combined Analysis of Phase III Trials Investigating Duration of Adjuvant Therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) Regimen for Patients with Stage III Colon Cancer: Trial Design and Current Status. Current colorectal cancer reports 2013; 9: 261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andre T, de Gramont A, Vernerey D, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol 2015; 33(35): 4176–87. [DOI] [PubMed] [Google Scholar]
- 18.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. 1 ed. New York: Springer-Verlag; 2000. [Google Scholar]
- 19.Harrington D, D’Agostino RB Sr., Gatsonis C, et al. New Guidelines for Statistical Reporting in the Journal. N Engl J Med 2019; 381(3): 285–6. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol 1998; 16(1): 295–300. [DOI] [PubMed] [Google Scholar]
- 21.Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015; 314(13): 1346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372(14): 1324–32. [DOI] [PubMed] [Google Scholar]
- 23.Shi Q, Gramont AD, Dixon JG, et al. Re-evaluating disease-free survival (DFS) as an endpoint versus overall survival (OS) in adjuvant colon cancer (CC) trials with chemotherapy +/− biologics: An updated surrogacy analysis based on 18,886 patients (pts) from the Accent database. Journal of Clinical Oncology 2019; 37(15_suppl): 3502-. [Google Scholar]
- 24.Pages F, Andre T, Taieb J, et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 25.Taieb J, Taly V, Vernerey D, et al. Analysis of circulating tumor DNA (ctDNA) from patients enrolled in the IDEA-FRANCE phase III trial: prognostic and predictive value for adjuvant treatment duration. Annals of Oncology 2019; 30: v851–v934. [Google Scholar]
- 26.Lapeyre-Prost A, Hug de Larauze M, Chibaudel B, et al. Feasibility of Capecitabine and Oxaliplatin Combination Chemotherapy Without Central Venous Access Device in Patients With Stage III Colorectal Cancer. Clin Colorectal Cancer 2016; 15(3): 250–6. [DOI] [PubMed] [Google Scholar]
- 27.Andre T, Vernerey D, Im SA, et al. Bevacizumab as adjuvant treatment of colon cancer: updated results from the S-AVANT phase III study by the GERCOR Group. Ann Oncol 2020; 31(2): 246–56. [DOI] [PubMed] [Google Scholar]
- 28.Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005; 16(4): 549–57. [DOI] [PubMed] [Google Scholar]
- 29.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005; 352(26): 2696–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


