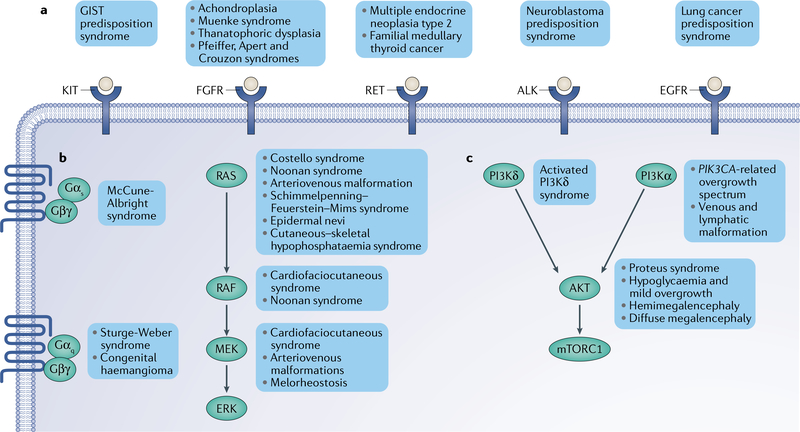
Fig. 4 |. The pathways of oncoprotein signalling in congenital disorders.
Most human oncoproteins are core components of the signalling pathways that regulate cell growth, division and proliferation. Among these, three major groups can be easily recognized: the receptor tyrosine kinase (RTK), PI3K-AKT and RAS-MAPK pathways. a | RTK pathway. Mutations in RTK genes often lead to ligand-independent activation of the receptor and signal through downstream pathways such as the PI3K, MAPK, signal transducer and activator of transcription (STAT) and SRC pathways. Most germline mutations in RTK genes lead to tumour predisposition syndromes. b | RAS-MAPK pathway. Mutations in genes in this pathway generally lead to cell cycle progression and proliferation. Activating mutations in the RAS gene isoforms (NRAS, HRAS and KRAS) are frequent in congenital disorders and cancer, but are mostly incompatible with life in the germline. Most germline syndromes include weak activating variants of the oncoproteins, such as the variants found cardiofaciocutaneous syndrome. Mutations in the genes encoding the trimeric G protein-associated GTPases GNAS, GNAQ and GNA11 cause syndromes characterized by their skin involvement, such as McCune-Albright syndome and Sturge-Weber syndrome. c | PI3K-AKT pathway. This is an important pathway that mainly regulates cell growth. Most mutations affecting PIK3CA, the PI3Ka isoform that generates phosphoinositide 3,4,5-trisphosphate, result in syndromes with severe overgrowth and vascular involvement. This is also true for AKT1 mutations in Proteus syndrome. Germline mutations in PIK3CD, the PI3Kδ isoform mostly expressed in lymphocytes, cause a syndrome characterized by immunodeficiency. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stromal tumour; mTORC1, mechanistic target of rapamycin complex 1.