Fig. 2 |. Cellular contributors to tissue-specific antitumour responses.
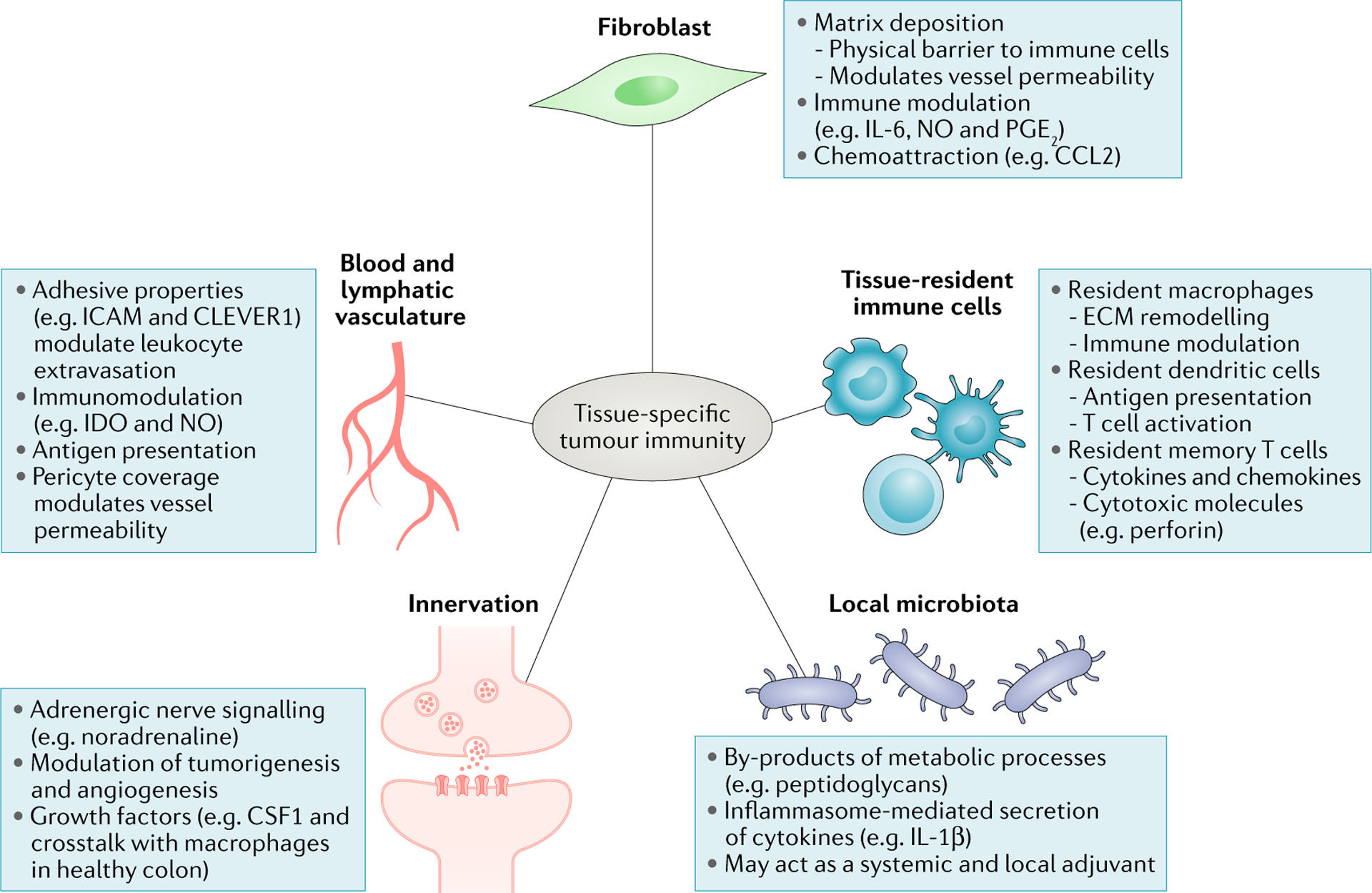
Blood vessels across anatomical sites can differentially control tumour immune infiltrate through distinct levels of expression of adhesion molecules, cohesiveness and pericyte coverage, regulating immune cell extravasation. While the differential impact of lymphatic vessels on the immune infiltrate across cancer types is poorly understood, it is now well established that lymphatics help shape immune responses by promoting tolerance to self-antigens, archiving antigen for later presentation and dampening effector immune responses214–217. Cancer-associated fibroblasts modulate antitumour immune responses by secreting chemokines, cytokines, growth factors and reactive oxygen species, as well as producing and remodelling the stromal matrix that serves both a guiding and a barrier function for immune cells. Emerging evidence shows that commensal bacteria can set the tone of antitumour immune responses both systemically and locally, for example, by stimulating immune cells to secrete inflammatory cytokines. Tissue-resident cells, which include macrophages, dendritic cells and memory T cells, contribute to shaping antitumour immune responses by acting directly on tumour cells or modulating infiltrating immune cells. Nerve cells, whose presence has been reported in a limited number of cancer types, influence tumour cell survival, angiogenesis and the function of immune cells such as macrophages by releasing neurotransmitters and growth factors. CCL2, CC-chemokine 1igand2; CLEVER1, common lymphatic endothelial and vascular endothelial receptor 1; CSF1, colony-stimulating factor 1; ECM, extracellular matrix; ICAM, intercellular cell adhesion molecule; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; NO, nitric oxide; PG E2, prostaglandin E2.
