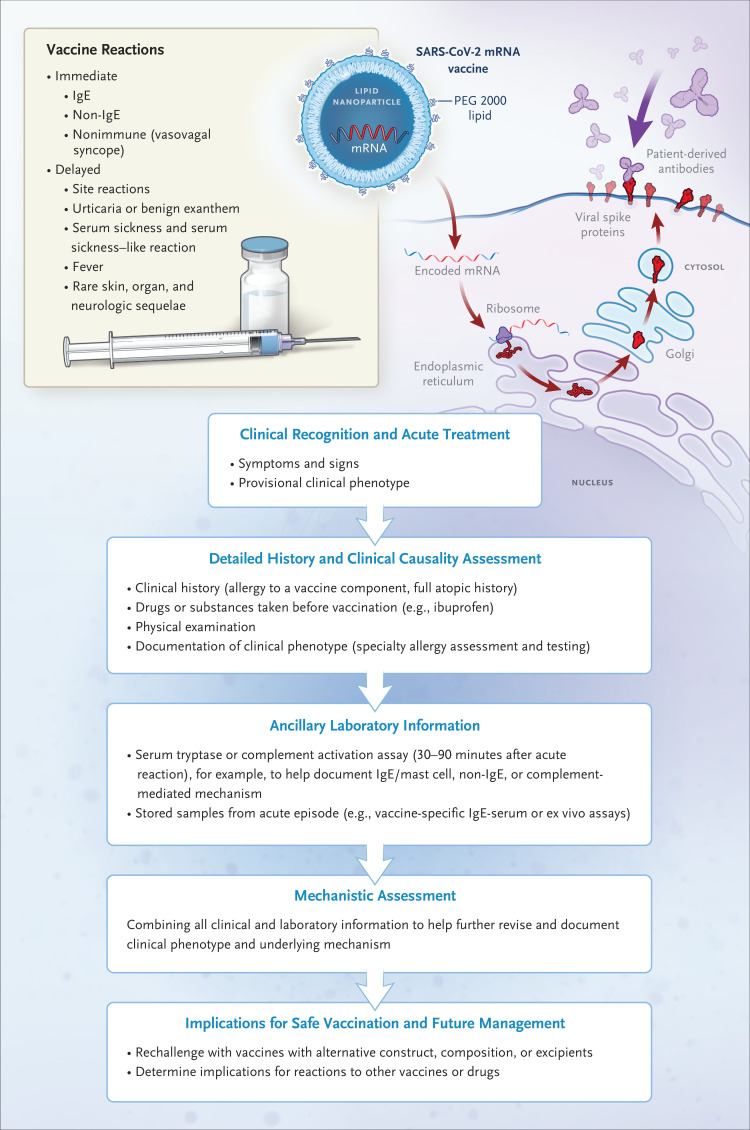
Figure 1. Assessing Reactions to Vaccines.
SARS-CoV-2 mRNA vaccines are built on the same lipid-based nanoparticle carrier technology; however, the lipid component of the Pfizer-BioNTech vaccine differs from that of the Moderna vaccine. Operation Warp Speed has led to an unprecedented response to the study of the safety and effectiveness of new vaccine platforms never before used in humans and to the development of vaccines that have been authorized for use less than a year after the SARS-CoV-2 viral sequence was discovered. The next few months could see the authorization of several such vaccines, and inevitably, adverse drug events will be recognized in the coming months that were not seen in the studies conducted before emergency use authorization. Maintenance of vaccine safety requires a proactive approach to maintain public confidence and reduce vaccine hesitancy. This approach involves not only vigilance but also meticulous response, documentation, and characterization of these events to heighten recognition and allow definition of mechanisms and appropriate approaches to prediction, prevention, and treatment. A systematic approach to an adverse reaction to any vaccine requires clinical recognition and appropriate initial treatment, followed by a detailed history and causality assessment. Nonimmune immediate reactions such as vasovagal reactions are common and typically manifest with diaphoresis, nausea, vomiting, pallor, and bradycardia, in contrast to the flush, pruritus, urticaria, angioedema, tachycardia, and laryngeal edema seen with anaphylaxis. Post-reaction clinical assessment by an allergist–immunologist that includes skin testing for allergy to components of the vaccine can be helpful. Use of other laboratory information may aid in clinical and mechanistic assessment and guide future vaccine and drug safety as well as management, such as rechallenge with alternative vaccines if redosing is required. A useful resource for searching the excipients of drugs and vaccines is https://dailymed.nlm.nih.gov/dailymed/. A useful resource for excipients in licensed vaccines is https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf.