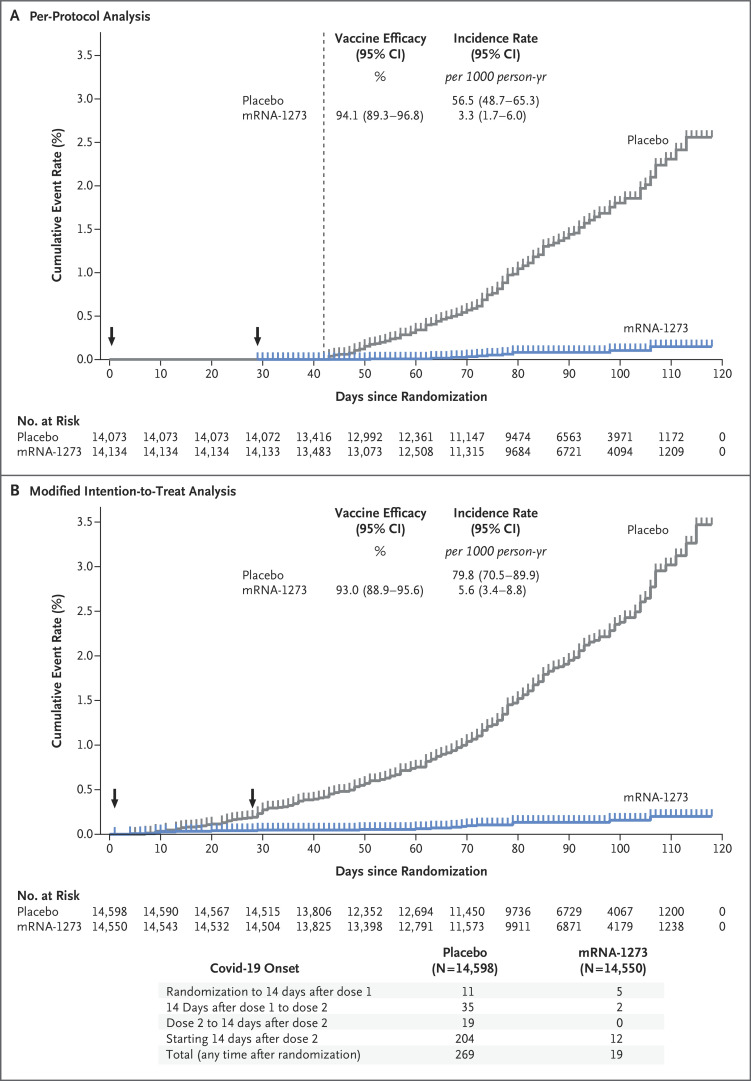
Figure 3. Vaccine Efficacy of mRNA-1273 to Prevent Covid-19.
Shown is the cumulative incidence of Covid-19 events in the primary analysis based on adjudicated assessment starting 14 days after the second vaccination in the per-protocol population (Panel A) and after randomization in the modified intention-to-treat population (Panel B) (see the Supplementary Appendix). The dotted line in Panel A indicates day 42 (14 days after vaccination 2), when the per-protocol follow-up began, and arrows in both panels indicate days 1 and 29, when injections were administered. Tick marks indicate censored data. Vaccine efficacy was defined as 1 minus the hazard ratio (mRNA vs. placebo), and the 95% confidence interval was estimated with the use of a stratified Cox proportional hazards model, with Efron’s method of tie handling and with treatment group as a covariate, with adjustment for stratification factor. Incidence was defined as the number of events divided by number of participants at risk and was adjusted by person-years. Symptomatic Covid-19 case accrual for placebo and vaccine in the modified intention-to-treat population is displayed (does not include asymptomatic cases of SARS-CoV-2 detected at the day 29 by nasopharyngeal swab).