Figure 1:
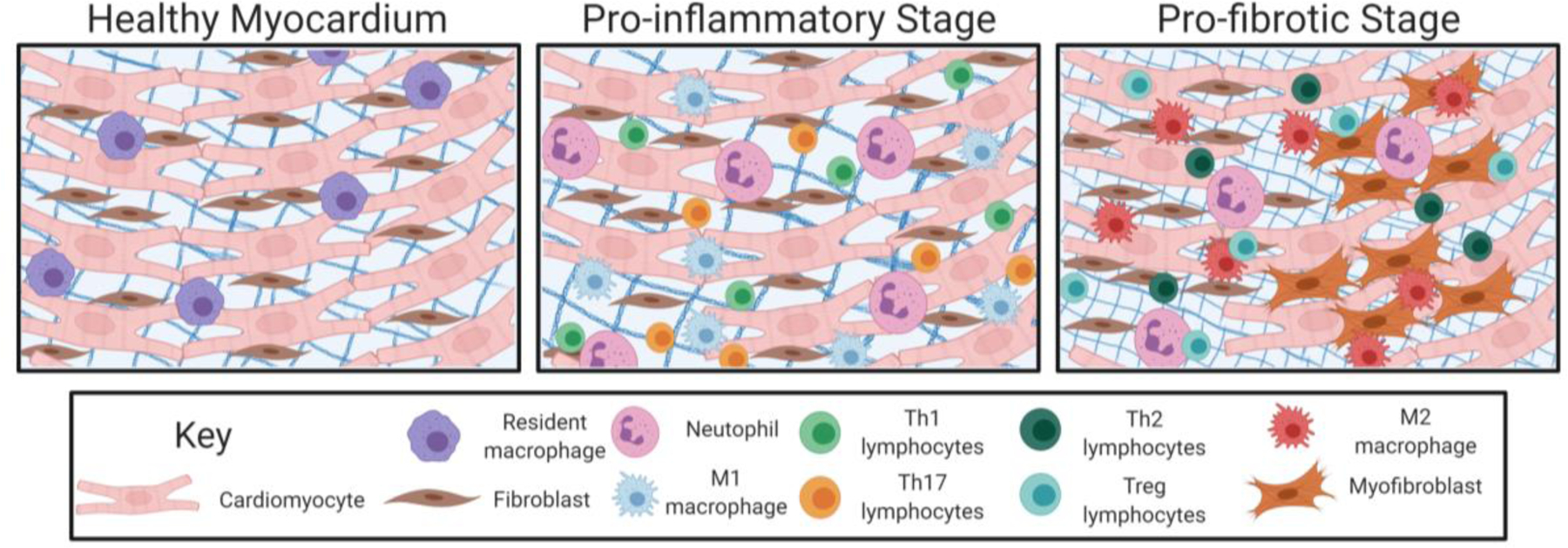
Cell involvement in healthy cardiac tissue and during the cardiac fibrotic response. Left Panel: In the healthy myocardium, healthy cardiomyocytes (red), resident fibroblasts (brown), and resident immune cells (i.e., macrophages; purple) reside. In this state, resident fibroblasts help to maintain a normal ECM through the production of collagens, elastin, and laminin. These resident cells also produce a balance of MMPs and TIMPs to help maintain a normal ECM in which these cells can reside. Center Panel: In the pro-inflammatory stages of cardiac fibrosis, DAMPs secreted by stressed or necrotizing cells lead to neutrophil (pink) invasion. This sets off an inflammatory cascade whereby macrophages are recruited and activated so that these cells take on an M1 pro-inflammatory phenotype (blue). In later stages of inflammation, Th1 (green) and Th17 (orange) lymphocytes are recruited and help to maintain activation of other inflammatory cells. Right Panel: As inflammatory signaling persists, macrophages take on an M2, pro-fibrotic phenotype (red). Th2 (dark green) and Treg (teal) lymphocytes also secrete anti-inflammatory and pro-fibrotic mediators leading to this switch toward increased fibrosis. Neutrophils (pink) also persist at this stage and directly secrete ECM components. Signaling from each these cell types activates pathways leading to fibroblast (brown) proliferation (left) and activation into myofibroblasts (dark orange; right). Myofibroblasts are contractile and secretory and are the predominant cell type secreting extracellular matrix proteins. The activation and accumulation of these cells results in a dense ECM (blue grid behind cells). These cells eventually differentiate into matrifibrocytes (not shown) to maintain a stable fibrotic scar. Created with BioRender.com
