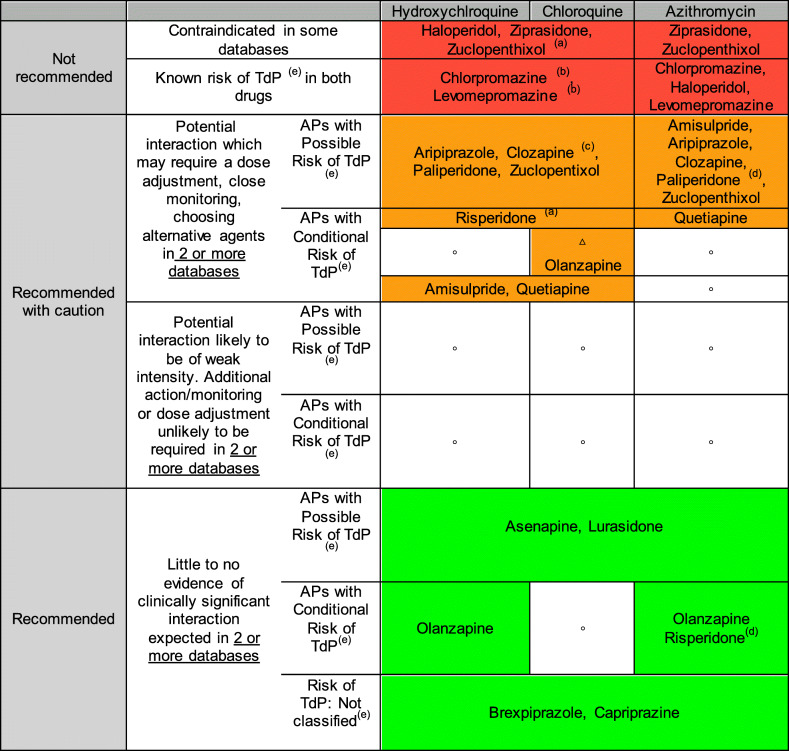
Table 2.
Drug-drug interaction between: antipsychotics and chloroquine/hydroxychloroquine/azithromycin
(a)Potential increased exposure of Aps, (b) Additional interaction: phenothiazides/antimalarial (increased serum concentration of phenothiazines), (c) Additional interaction: Risk Hematological toxicity, (d) Paliperidone and Risperidone (P-glycoprotein/ABCB1 Substrates) and Azithromycin (P-glycoprotein/ABCB1 inhibitors), (e) Torsade de Pointes (TdP) Risk Categories by QTDrugs de CredibleMeds®: Known Risk of TdP (these drugs prolong the WT interval and are clearly associated with a known risk of TdP, even when taken as recommended), Possible Risk of TdP (these drugs can cause QT prolongation but currently lack evidence for risk of TdP when taken as recommended), Conditional Risk of TdP (these drugs are associated with TdP but only under certain conditions of their use (e.g., excessive dose, in patients with conditions such as hypokalemia, or when taken with interacting drugs) or by creating conditions that facilitate or induce TdP (e.g., by inhibiting metabolism of QT-prolonging drugs or by causing an electrolyte disturbance that induce TdP), Not classified (this drug has been reviewed but the evidence available at this time did not result in a decision for it to be placed in any of the four QT risk categories. This is not an indication that this drug is free of a risk of QT prolongation or Torsade de Pointes since it may not have been adequately tested for these risks in patients)
△There is not enough information to assign the antipsychotic to any of the previous risk rating categories; the recommendations reflect the most conservative approach. This recommendation to be taken with caution; ° No antipsychotics in this category