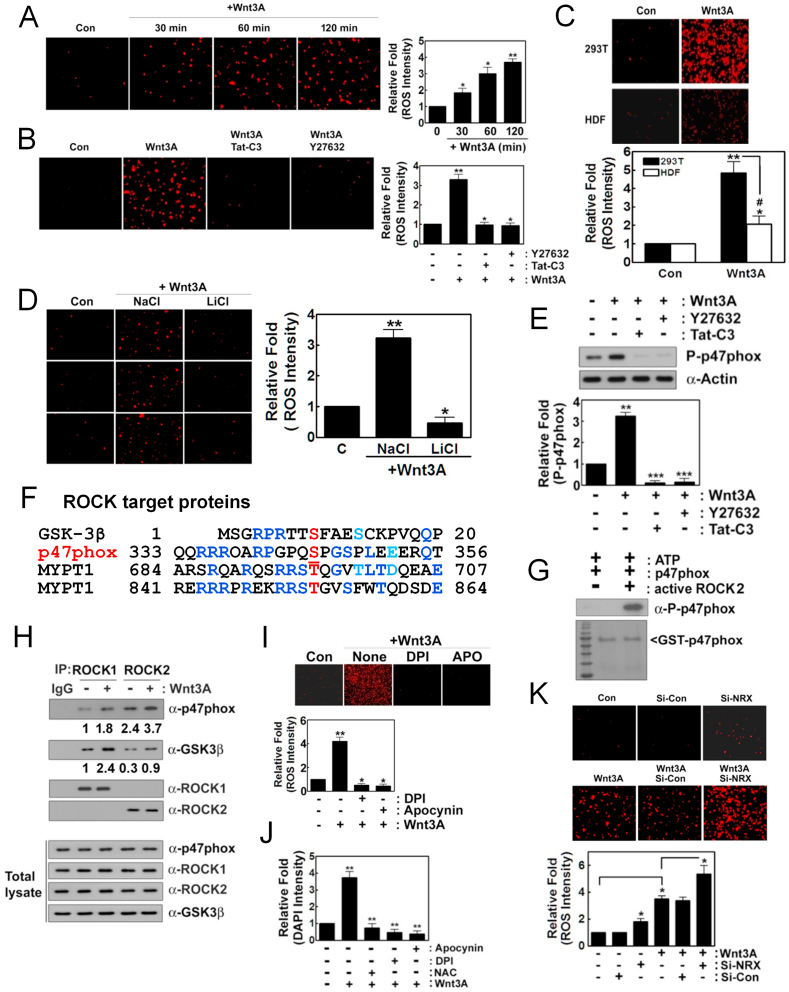
Fig. 1.
Wnt3A induces superoxide production through ROCK2 and p47phox. (A) HEK293T cells (2–5x104) were cultured in DMEM 5% FBS and 1% P/S. For Wnt3A signaling, they were starved for 12 h without serum and treated with Wnt3A (30 ng/ml) for the indicated periods. Produced superoxide was visualized with hydroethidine (1 μM) for 5–15 min, detected using a fluorescence microscope (Axiovert 200, Carl Zeiss). (B) Cells were pretreated with Tat-C3 (0.1 μg/ml) and Y27632 (10 μM) for 1 h and then activated with Wnt3A (30 ng/ml) for 2 h and superoxide was detected with hydroethidine as in (A). (C) Human dermal fibroblast (HDF) and HEK293T cells (2–5x104) were treated with Wnt3A (30 ng/ml) for 2 h and the produced superoxide was detected as in (A). (D) Cells were pretreated with LiCl (20 mM) or NaCl (20 mM) for 1 h and then treated with Wnt3A (30 ng/ml) for 2 h with produced superoxide detected as above. (E) Cells were pretreated with Tat-C3 (0.1 μg/ml), Y27632 (10 μM) for 1 h and then treated with Wnt3A (30 ng/ml) for 2 h. P-Ser345 p47phox level changes were determined with western blotting. (F) Amino acid sequences of GSK-3β, p47phox, myosin phosphatase 1 (MYPT1) were compared. (G) Recombinant purified GST-p47phox (0.2 μg) protein was incubated with an active fragment of purified ROCK2 protein (0.1 μg), ATP (0.1 mM) and MgCl2 (1 mM) for 30 min at RT. P-Ser345 p47phox was detected by western blotting. (H) HEK293T cells were stimulated with Wnt3A and ROCK1 and ROCK2 were immunoprecipitated and co-precipitated p-47phox and GSK-3β were determined by western blotting. (I, J) Cells were pretreated with DPI (10 μM) and apocynin (10 μM) for 1 h and then treated with Wnt3A (30 ng/ml) for 2 h and produced superoxide was detected as above (I), and cell proliferation was determined using DAPI staining (J). (K) HEK293T cells were transfected with si-control and si-nucleoredoxin (NRX) (10 nM) for 72 h, then treated with Wnt3A (30 ng/ml) for 2 h with the produced superoxide detected as described above.