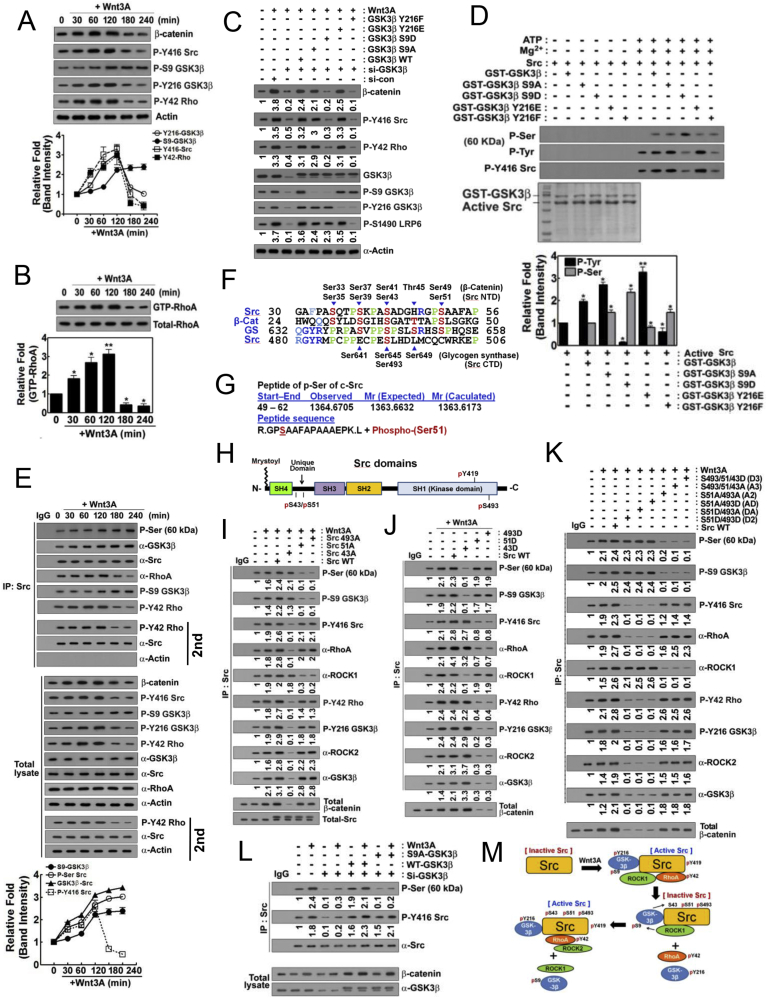
Fig. 3.
P-Tyr216 GSK-3β stimulates β-catenin in early period and then p-Ser9 GSK-3β attenuates β-catenin through Src phosphorylation in late period of Wnt3A stimulation. (A) HEK293T cells were stimulated by Wnt3A in time-dependent manner and indicated proteins were determined. (B) RhoA-GTP levels were determined using RBD-beads pull-down assay. (C) HEK293T cells were transfected with si-GSK3β and reconstituted with GSK-3β WT, S9A, S9D, Y216E and Y216F. Cells were stimulated by Wnt3A (30 ng/ml) for 2 h and indicated proteins were determined. (D) Recombinant GST-GSK-3β WT, S9A, S9D, Y216E and Y216F proteins were incubated with Src protein in the presence of ATP (20 μM) and MgCl2 (25 mM) for 60 min and p-Ser and p-Tyr416 Src were detected with western blotting. (E) HEK293T cells were stimulated with Wnt3A (30 ng/ml) for 2 h and Src was immunoprecipitated and then indicated proteins were detected. (F) Amino acids sequences of N- (NTD) and C-terminal domains (CTD) of Src, β-catenin and glycogen synthase (GS) were compared. Identical amino acids were indicated in blue and similar ones were noted in cyan blue. Specific Ser candidate residues to be phosphorylated were noted in red. Particularly identical proline residues were noted in green. (G) GST-GSK-3β S9D and Src proteins were incubated with ATP/MgCl2, the SDS-PAGE was performed, and Src protein was analyzed to identify phosphorylation site by using MALDI-TOF. Ser51 was identified to be phosphorylation site. (H) Domains of Src including SH4, Unique domain (UD), SH3, SH2, and SH1 was presented. P-Ser51 and p-Ser43 were localized in UD and p-Ser493 was localized in SH1 domain. (I) Cells were transfected with Src S493A, S51A, S43A mutants and WT (wild type), then Src was immunoprecipitated and then p-Ser and p-Tyr416 Src, p-S9 GSK-3β, p-Tyr216 GSK-3β, p-Tyr42 RhoA, ROCK1, and ROCK2 were immunoblotted. (J) Cells were transfected with Src S493D, S51D, S43D mutants, and WT (wild type), then Src was immunoprecipitated and then p-Ser and p-Tyr416 Src, p-S9 GSK-3β, p-Tyr216 GSK-3β, p-Tyr42 RhoA, ROCK1 and ROCK2 were immunoblotted. (K) Cells were transfected with Src A3 (S493A/S51A/S43A), D3 (S493D/S51D/S43D), A2 (AA: S493A/S51A), D2 (DD: S493D/S51D), AD (S493A/S51D) and DA (S493A/S51A), and WT (wild type) and Sc was immunoprecipitated and then p-Ser and p-Tyr416 Src, p-S9 GSK-3β, p-Tyr216 GSK-3β, p-Tyr42 RhoA, ROCK1 and ROCK2 were immunoblotted. (L) si-GSK-3β, GSK-3β WT and GSK-3β S9A were transfected and treated with Wnt3A for 2 h. Src was immunoprecipitated and p-Ser was immunoblotted. (M) Tentative model for p-Ser9 GSK-3β to phosphorylate Ser493, Ser51, and Ser43 residues of Src was presented. p-Ser493 and p-Ser51 Src are inactive forms, but p-Ser43 Src is an active form. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)