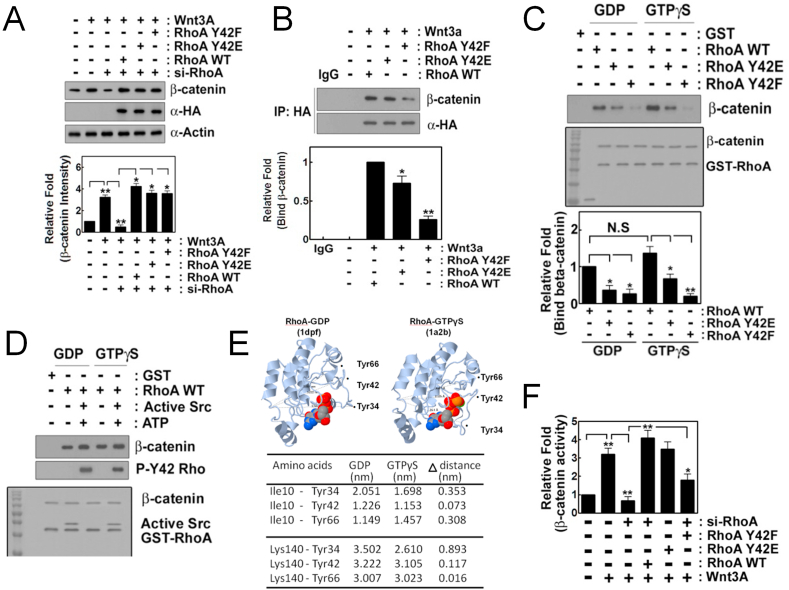
Fig. 5.
P-Tyr42 RhoA interacts with β-catenin. (A) HEK293T cells (2–5x105) were transfected with si-RhoA (10 nM) for 72 h and were then transfected with HA-RhoA WT, Y42E and Y42F (4 μg plasmid DNA) for 24 h. Cells were treated with Wnt3A (30 ng/ml) for 2 h and β-catenin was detected by western blotting. (B) HA-containing RhoA was immunoprecipitated and β-catenin co-immunoprecipitated with RhoA was detected with western blotting. (C) Recombinant purified GST-RhoA (0.1 μg/50 μl) that was preloaded with GDP or GTPγS was incubated with β-catenin (0.1 μg/50 μl) for 30 min at RT in vitro. β-Catenin associated with GST-RhoA was detected with western blotting. (D) Recombinant purified GSH beads-GST-RhoA (0.1 μg/50 μl) that was preloaded with GDP or GTPγS was incubated with purified Src (0.1 μg/50 μl), ATP (20 μM) and MgCl2 (25 mM) for 30 min to phosphorylate Tyr42 of RhoA and then β-catenin (0.1 μg/50 μl) was added and incubated more for 1 h. β-Catenin associated with GSH beads-GST-RhoA was detected with western blotting. (E) Three dimensional structures of RhoA-GDP and -GTPγS and Tyr34, Tyr42 and Tyr66 were indicated. Distances from Ile10 or Lys140 to Tyr34, Tyr42 and Tyr66 of RhoA in the presence of GDP or GTPγS were measured using Proteopia site. (F) HEK293T cells were transfected with si-RhoA for 72 h, then with RhoA WT, Y42E and Y42F along with β-catenin promoter-luciferase DNA (2 μg) for 24 h and luciferase activity was measured.