Summary
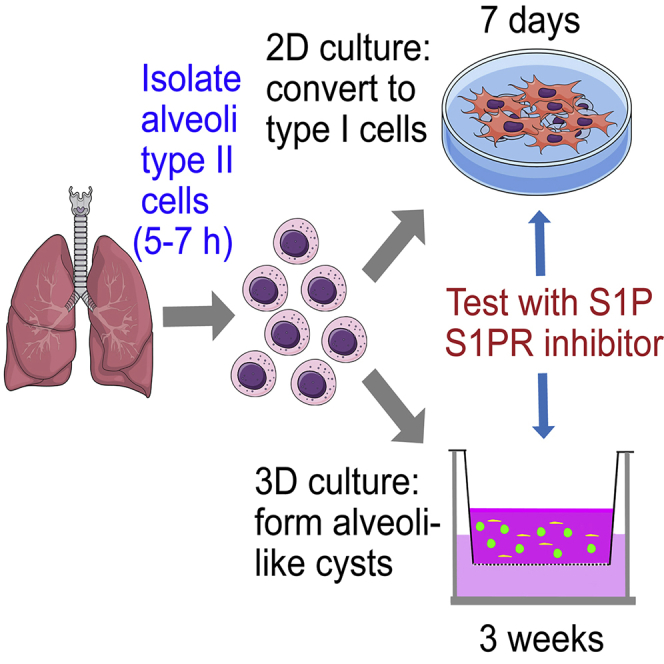
Alveolar type II cells (AT2) are stem cells of lung epithelium. To study these cells, we developed protocols to isolate primary AT2 and test the effects of potential niche factors in regulating the AT2 progenitor functions. AT2 freshly isolated from mouse lungs are grown in 2D or 3D culture. AT2 are able to differentiate into alveolar type I cells (AT1) in these conditions. S1P or an inhibitor of S1P signaling is added in culture to modulate AT2 to AT1 transition.
For complete details on the use and execution of this protocol, please refer to Chen et al. (2020).
Subject Areas: Cell culture, Cell isolation, Cell-based Assays, Model Organisms, Stem Cells, Cell Differentiation
Graphical Abstract
Highlights
-
•
A protocol for isolating mouse lung alveolar type II cells
-
•
Grow type II cells in 2D culture and observe their differentiation to type I cells
-
•
Grow type II cells in 3D culture and generate alveolospheres
-
•
Test the role of sphingosine-1-phosphate signaling in type II-to-I cell transition
Alveolar type II cells (AT2) are stem cells of lung epithelium. To study these cells, we developed protocols to isolate primary AT2 and test the effects of potential niche factors in regulating the AT2 progenitor functions. AT2 freshly isolated from mouse lungs are grown in 2D or 3D culture. AT2 are able to differentiate into alveolar type I cells (AT1) in these conditions. S1P or an inhibitor of S1P signaling is added in culture to modulate AT2 to AT1 transition.
Before you begin
All studies using animals have been approved by the Institutional Animal Care Committee of the University of Illinois at Chicago.
Prepare type II cell lineage traced mice
Timing: 2 weeks
-
1.Prepare tamoxifen solution
-
a.Make 20 mg/mL tamoxifen stock solution in corn oil. Incubate the stock solution at 45°C for 6–7 h and vortex occasionally to dissolve the tamoxifen powder.
-
b.Aliquot and store the stock solution at −20°C for several months.
-
a.
Note: Tamoxifen is light-sensitive and should be wrapped in foil or kept in dark.
-
2.
Inject tamoxifen to CreER induced lineage tracing mice: Adult mice at 8–16 weeks of age are intraperitoneally (i.p.) injected with tamoxifen every other day at 0.2 mg/g for 4 times. Mice are used 4–7 days after the last injection.
Note: AT2 cell isolation done in mice at this age range does not show difference in yield and purity between sex. However, the sex of the mice should always be taken into consideration when designing the experiments.
-
3.
For type II cell lineage labeling, we used SpC-CreER/ROSA-Tomato mice or SpC-CreER/mTmG mice (Barkauskas et al., 2013; Rock et al., 2011) (Chen et al., 2020).
Note: The reason that we use AT2 lineage tracing mice is because by this it is easier for us to visualize AT2 in culture, especially 3D culture. If other approaches are available to visualize the cells, then lineage tracing mice are not required.
Prepare reagents for type II cell isolation and culture
Timing: 1–2 days
-
4.Prepare for type II cell isolation:
-
a.Prepare other reagents and materials for isolating type II cells:
-
i.HEPES-buffered DMEM: See “Materials and equipment” for details.
-
ii.Dispase (50 units/mL, approximately 2.5 mL needed per mouse)
-
iii.70 μm cell strainer ( Corning Falcon)
-
iv.20 μm nylon gauze (Fisher)
-
v.DNase I (Sigma)
-
i.
-
b.Coat tissue culture dishes with CD45 and CD32 antibodies. Coat 100-mm tissue culture plates with 42 μg CD45 and 16 μg CD32 in 7 mL PBS for 24–48 h at 4°C. Before use, wash the plates twice with PBS and once with HEPES-buffered DMEM.
-
a.
-
5.Prepare for 2D type II cell culture:
-
a.Autoclave round glass coverslips for 20 min in glass beakers to sterilize. 15 mm round glass coverslips are used in 24-well plates and 18 mm coverslips are used in 12-well plates.
-
b.Let the coverslips cool down to 20°C–25°C.
-
c.Used sterile forceps to transfer coverslips to culture wells.
-
a.
-
6.Prepare for 3D culture:
-
a.Grow Mlg cells (mouse lung fibroblasts, ATCC) (Lee et al., 2014) in Mlg cell culture medium (See “Materials and equipment” for details). The cells usually take ~3 days after thawing to reach 80% confluency. Subculturing the cells at 1:2 to 1:4 ratios when necessary.Note: we used cells at passage 6 or less.
-
b.Prepare other reagents and materials for 3D culture
-
i.Matrigel: When opening a new bottle (5 mL or larger volume) of Matrigel, submerge the bottle in ice or keep at 4°C for 5 to 18 h to thaw slowly. After the whole bottle is completely thawed, use cold tips to aliquot Matrigel into cold sterile 1.5 mL tubes and keep the aliquots at −20°C. Keep the Matrigel on ice during aliquoting.
-
ii.3D culture media (for 3D co-culture of type II cells and Mlg): See “Materials and equipment” for details.
-
i.
-
c.Equilibrate 6.5 mm Transwell polycarbonate membrane inserts (0.45 μm pore size, Corning) in 24 well plates. Add 0.6 mL 3D culture media to the wells and 100 μL of media inside the insert. Keep the plates in a 37°C cell culture incubator with 5% CO2 for 1–18 h.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| pro-Sp-C (rabbit, 1:500) | Millipore | Cat# AB3786, RRID:AB_91588 |
| T1α (hamster, 1:50) | Developmental Studies Hybridoma Bank | Cat# 8.1.1, RRID:AB_531893 |
| RFP (rabbit, 1:200) | Rockland Immunochemicals | Cat# 600-401-379, RRID:AB_1182807AB_2209751 |
| GFP (chicken, 1:500) | Aves Laboratories | Cat# GFP-1020, RRID:AB_10000240 |
| HopX (mouse, 1:50) | Santa Cruz Biotechnology | Cat# sc-398703, RRID:AB_2687966 |
| Ager (rat, 1:50) | R&D Systems | Cat# MAB1179, RRID:AB_2289349 |
| Biotin-EpCAM (CD326, for cell isolation, rat, 1:50) | Thermo Fisher Scientific | Cat# 13-5791-82, RRID:AB_1659713 |
| CD45 (rat, 42 μg/plate) | BioLegend | Cat# 103101, RRID:AB_312966 |
| CD32 (rat, 16 μg/plate) | BioLegend | Cat# 156402, RRID:AB_2783133 |
| Streptavidin Particles Plus – DM (RUO) | BD Biosciences | Cat# 557812, RRID:AB_10050580 |
| Chemicals, peptides, and recombinant proteins | ||
| Tamoxifen | Sigma-Aldrich | Cat# T5648 |
| Dispase | Corning | Cat# 354235 |
| JTE-013 | Cayman Chemical | Cat# 10009458 |
| Matrigel | Corning | Cat# 354230 |
| SYBR green | Roche | Cat# 04913914001 |
| Sphingosine-1-phosphate | Cayman Chemical | Cat# 62570 |
| DNase I | Sigma | Cat# D5025 |
| Bovine serum albumin (BSA) | MP Biomedicals | Cat# 9048-46-8 |
| Bovine serum albumin (BSA, fatty acid free) | Sigma | Cat# A8806 |
| Trypan blue | Lonza | Cat# 17-942E |
| Paraformaldehyde | Sigma | Cat# P6148 |
| Agarose | Dot Scientific | Cat# CGLE-500 |
| DAPI | Sigma | Cat# D9542 |
| Dextran-coated charcoal | Sigma | Cat# C6241 |
| Dulbecco's modified Eagle’s medium (DMEM) | Corning | Cat# 10-013-CV |
| HEPES | Thermo Fisher Scientific | Cat# 15630106 |
| Fetal bovine serum (FBS, heat inactivated) | Hyclone | Cat# SH30910.03 |
| Penicillin-streptomycin | Thermo Fisher Scientific | Cat# 15140122 |
| Gentamicin | Sigma | Cat# G1397 |
| Amphotericin | Thermo Fisher Scientific | Cat# 15290018 |
| Insulin-transferrin-selenium (ITS) | Sigma | Cat# I1884-1VL |
| Antibiotic-antimycotic (Anti-Anti) | Thermo Fisher Scientific | Cat# 15240062 |
| Dulbecco's modified Eagle’s medium: nutrient mixture F-12 (DMEM/F-12) | Thermo Fisher Scientific | Cat# 4192001 |
| Corning collagen I, rat | Corning | Cat# 354236 |
| RBC lysis buffer | eBioscience | Cat# 00-4333-57 |
| Eagle's minimum essential medium (EMEM) | ATCC | Cat# 30-2003 |
| Critical commercial assays | ||
| RNeasy mini kit | Qiagen | Cat# 74104 |
| Experimental models: cell lines | ||
| Mlg mouse lung fibroblast cells | ATCC | CCL-206 |
| Experimental models: organisms/strains | ||
| Mouse: SpC-CreER (Sfptctm(cre/ERT2)Blh) | From Dr. Brigid Hogan, Duke University | N/A |
| Mouse: ROSA-Tomato (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J) | The Jackson Laboratory | Stock# 007914 |
| Mouse: mTmG (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J) | The Jackson Laboratory | Stock# 007576 |
| Software and algorithms | ||
| ZEN v2.3 | Zeiss Microscopy | https://www.zeiss.com/microscopy/us/products/microscope-software/zen.html |
| Other | ||
| Corn oil | Mazola | N/A |
| 70 μm cell strainer | Corning Falcon | Cat# 352350 |
| 20 μm nylon gauze | Fisher Scientific | Cat# 08-801-15 |
| 100 mm tissue culture plate | Dot Scientific | Cat# 667621 |
| Phosphate buffered saline | Sigma | Cat# P3813 |
| 15 mm round glass coverslip | VWR | Cat# CLS-1760-015 |
| 18 mm round glass coverslip | VWR | Cat# 72229-01 |
| 6.5 mm Transwell inserts (0.45 μm pore size) | Corning | Cat# 3413 |
| Glass slides | Fisher | 12-550-15 |
| 24-well cell culture plate | Denville | Cat# T1024 |
| MagRack (magnetic rack) | Sigma | Cat# GE28-9489-64 |
| Cytospin cytocentrifuge | Thermo Fisher Scientific | N/A |
| MoFlo Astrios cell sorter | Beckman Coulter | N/A |
Materials and equipment
Tables for solution and/or buffer recipes
| HEPES-buffered DMEM (for isolating type II cells and 2D culture) | Final concentration | Amount |
|---|---|---|
| DMEM | – | 450 mL |
| HEPES buffer (1 M) | 25 mM | 12.5 mL |
| FBS (heat inactivated) | 10% | 50 mL |
| Pen-strep | 100 U/mL penicillin, 100 μg/mL streptomycin | 5 mL |
| Antibiotic-antimycotic | 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B | 5 mL |
| Gentamicin and amphotericin | 10 μg/mL gentamicin 2.5 μg/mL amphotericin |
5 mL |
| Total | n/a | 500 mL |
Keep sterile and store at 4°C for 4 weeks.
| 3D culture media (for 3D co-culture of type II cells and Mlg) | Final concentration | Amount |
|---|---|---|
| DMEM/F-12 | – | 450 mL |
| HEPES buffer (1 M) | 25 mM | 12.5 mL |
| FBS (heat inactivated) | 10% | 50 mL |
| Pen-strep | 100 U/mL penicillin, 100 μg/mL streptomycin | 5 mL |
| Antibiotic-Antimycotic | 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B | 5 mL |
| Gentamicin and amphotericin | 10 μg/mL gentamicin 2.5 μg/mL amphotericin |
5 mL |
| ITS, insulin, transferrin, and selenium | 1× | 5 mL |
| Total | n/a | 500 mL |
Keep sterile and store at 4°C for 4 weeks.
| Mlg cell culture medium | Final concentration | Amount |
|---|---|---|
| Eagle's minimum essential medium (EMEM) (ATCC 30-2003) | – | 500 mL |
| FBS (heat inactivated Hyclone) | 10% | 50 mL |
| Pen-strep | 100 U/mL penicillin, 100 μg/mL streptomycin | 5 mL |
| Antibiotic-antimycotic | 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B | 5 mL |
| Gentamicin and amphotericin | 10 μg/mL gentamicin 2.5 μg/mL amphotericin |
5 mL |
| Total | n/a | 550 mL |
Keep sterile and store at 4°C for 4 weeks.
Step-by-step method details
Isolation and purification of mouse lung alveolar type II cells
Timing: 5–7 h for isolation, 2–3 days to determine the purity
In this step, we will first isolate alveolar type II cells from mouse lungs. Next, we will purify the cell population by magnetic beads sorting or by flow cytometry sorting.
-
1.
Isolate type II cells from mouse lungs (See Methods Video S1)
-
a.Perfuse the lung with 10 mL PBS at 20°C–25°C via the pulmonary artery (Figure 1A).
-
b.Instill 2 mL dispase into the lung through trachea followed by 0.5 mL melted agarose (2% in PBS) (~45°C) (Figure 1A).
-
c.Immediately cover the lung with ice for 2 min to let the agarose solidify and seal the upper airways.Note: 0.5 mL agarose (2% in PBS) (~45°C) injected after the dispase seal the upper airways and block most of the airway cells from dissociation. In this way, we minimize the contamination of club cells in our type II cell isolation.
-
d.Tie the trachea and remove the lung from the mouse using forceps. Incubate the lung in 0.5 mL dispase in a 15 mL tube for 45 min at 20°C–25°C.
-
e.Transfer the lung to a 60 mm tissue culture dish containing 5 mL of HEPES-buffered DMEM with 100 unit/mL DNase I. Gently tease the lung tissue into small pieces (smaller than 2 mm3) from the bronchi. Cut 2–3 mm from the tip of a 1 mL pipette tip and use it to pipette the tissue pieces up and down to further dissociate the cells. Incubate the cells at 20°C–25°C for 10 min.
-
f.Filter the cell suspension through a 70 μm cell strainer and then nylon gauze (20 μm pore size) to get single cell suspension in a 15 mL centrifuge tube.Note: After the serial filtration, most cells left in the mixture should be single cells. To minimize the cell loss, after the 5 mL cells are filtered through the 70 μm cell strainer, 1 mL of additional HEPES-buffered DMEM can be added to the 60 mm tissue culture dish to collect residual cells and filter through the same cell strainer to be combined with the rest of the cells.
-
g.Centrifuge cells at 130 × g for 8 min at 4°C in a centrifuge suitable for 15 mL centrifuge tubes.Note: We used a relatively low speed (130 × g). In this way, most of the type I cells will be removed.
-
h.Re-suspend cells in 5 mL HEPES-buffered DMEM.
-
i.Place cells in tissue culture plates coated with CD45 and CD32 antibodies.
-
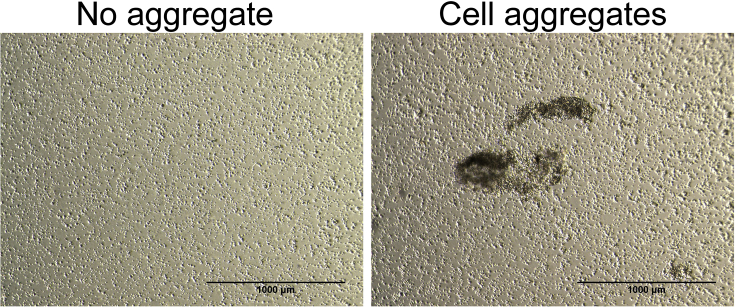
j.Incubate cells at 37°C, 5% CO2 for 1 h.Note: Occasionally, cell aggregates are seen after 1 h of incubation (Figure 2). In this case, add an additional aliquot of DNase I directly to the plate. Gently rock and swirl the plate to make it evenly disperse into the medium and incubate the plate for another 10 min. Proceeding with cell aggregates would compromise the purity and yield of the isolation.
-
k.Transfer suspending cells from the plate into a 15 mL tube and pellet the cells by centrifugation at 130 × g for 8 min.
-
l.Re-suspend cells in 1 mL RBC lysis buffer and transfer to 1.5 mL centrifuge tubes. Incubate cells on ice for 1 min.Note: If the lung is perfused well, one-minute incubation should be enough to remove all red blood cells. Longer incubation could result in lower type II cell viability. In case 1 min is not enough to remove all blood cells, the procedure can be repeated one more time.
-
m.Spin down cells at 400 × g for 3 min at 4°C and wash cells in PBS twice.
Methods Video S1. Lung perfusion and intratracheal instillation, refer to step 1Download video file (37.4MB, flv) -
a.
Note: The cell yield at this stage is ~3 × 106 per mouse.
-
2.Purify the cells with magnetic beads sorting
-
a.Re-suspend cells in 250 μL of FACS buffer (PBS + 2% FBS).Note: In experiments with S1P treatment, use charcoal stripped FBS instead of regular FBS in FACS buffer. To make charcoal stripped FBS, add 1 g dextran-coated charcoal to 50 mL FBS and shake gently on a shaker at 4°C for 16–18 h. After incubation, remove the charcoal by first centrifuging it down at 2,000 × g for 5 min at 4°C and then filtering the supernatant through a 70 μm cell strainer and a 0.2 μm syringe filter sequentially.
-
b.Add 4 μL of biotin conjugated anti-EpCAM antibody to the cells and incubate for 45 min on a rotator at 4°C.
-
c.Spin down the cells at 400 × g for 3 min and then re-suspend in 1 mL FACS buffer to wash. Repeat the wash twice and after the last wash, re-suspend cells in 500 μL FACS buffer. Add 20 μL of streptavidin conjugated magnetic beads and incubate for 30 min at 4°C with rotation.
-
d.Wash cells in 1 mL of MACS buffer (1× PBS, 0.5% BSA, 2 mM EDTA-pH 7.2) twice, re-suspend in 500 μL MACS buffer and arrange tubes in a magnetic rack (Sigma GE28-9489-64).Note: We recommend making fresh FACS and MACS buffer each time for the isolation since it is hard to keep the buffers sterile over time.Note: In experiments with S1P treatments, use fatty acid free BSA instead of regular BSA in MACS buffer.
-
e.Let the cells bind for 8 min on ice and remove negative/unbound fraction with a 1 mL pipette.
-
f.Re-suspend positive/bound fraction in 1 mL of MACS buffer.
-
g.Let the cells bind for 8 min and remove negative/unbound fraction with a 1 mL pipette.
-
h.Repeat step f and g one more time.
-
i.Stain the cells with trypan blue dye and determine viability and yield. Cell viability is ~70% and yield is ~5 × 105 per mouse at this stage.
-
a.
-
3.Determine the purity of isolated type II cells
-
a.Centrifuge cells onto glass slides using a cytospin centrifuge: re-suspend 2–5 × 104 cells in 150 μL PBS. Spin for 5 min at 500 rpm (28 × g).
-
b.Dry the cells for 5 min at 20°C–25°C. Circle the cells on slides with a hydrophobic pen.
-
c.Fix cells in 4% paraformaldehyde (PFA) at 20°C–25°C for 15 min. Wash slides in PBS for 3 times.
 Pause point: Cytospin slides can be stored in PBS at 4°C for about a week.
Pause point: Cytospin slides can be stored in PBS at 4°C for about a week. -
d.Stain cells with antibody against type II cell marker Sp-C (Kalina et al., 1992; Korfhagen et al., 1990) using general immunofluorescence staining procedures. (Figure 1B)
-
a.
Note: We use a rabbit anti pro-Sp-C antibody (see details in Key resources table) and fluorescence conjugated anti-rabbit secondary antibodies in the immunofluorescence staining. See (Chen et al., 2020) and (Liu et al., 2015) for more details.
Optional: The purity of isolated type II cells can also be determined by a PAP assay (Dobbs, 1990).
Note: By magnetic beads purification, we got ~500,000 cells each mouse. The purity is 95.6% ± 0.04%.
CRITICAL: 0.5 mL agarose (2% in PBS) (~45°C) is injected after the dispase. In this way, most of the airway club cells are blocked from dissociation and thus the contamination by club cells is minimum.
CRITICAL: In the first several centrifugations, we used a relatively slow speed (130 × g). In this way, most of the type I cells will be removed.
Pause point: After fixation, cytospin slides can be stored in PBS at 4°C for about a week.
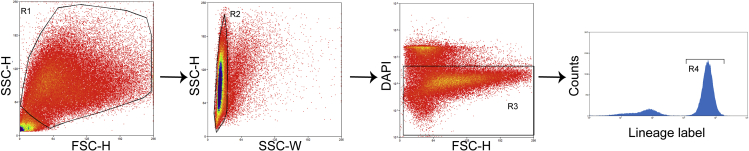
Alternatives: Type II cells can also be purified by FACS sorting. In FACS sorting, type II cells are isolated by fluorescent lineage labeling (Tomato for SpC-CreER/ROSA-Tomato mice or GFP for SpC-CreER/mTmG mice) and DAPI is added to exclude the dead cells. Sorting was done using the Beckman Coulter MoFlo Astrios Cell Sorter in the flow cytometry core at the University of Illinois at Chicago (Figure 3).
Figure 1.
Isolating type II cells from mouse lung.
(A) Dissecting the mouse lung for type II cell isolation. Step 1: perfuse the lung with 10 mL PBS via the pulmonary artery. Step 2: inject 2 mL dispase through the trachea followed by 0.5 mL agarose (2% in PBS).
(B) Isolated type II cells were fixed on glass slides by cytospin centrifugation and stained with antibody to type II cell marker Sp-C. Scale bar, 40 μm.
Figure 2.
Cell aggregates in step 1J
Scale bar, 1,000 μm.
Figure 3.
Gate for FASC sorting of type II cells
Type II cells were sorted with gates for forward, side scatter, DAPI, and lineage markers (depending on the lineage labeling lines used; e.g., Tomato for SpC-CreER/ROSA-Tomato mice or GFP for SpC-CreER/mTmG mice).
2D culture of isolated type II cells
Timing: 4–7 days
In this part, we culture the isolated type II cells in a 2D culture system. Cells are cultured on coverslips for antibody staining or directly in culture plates for qRT-PCR experiments. In the 2D condition, type II cells undergo transition into type I-like cells in 4–7 days.
-
4.Culture type II cells in 24-well plates
-
a.Place a 15 mm round glass coverslip in each well to be used of a 24-well plate.
-
b.Coat coverslips or wells with 250 μL 0.2% gelatin (prepared in Milli Q water, heated to dissolve and sterile filtered). Coat for 1–18 h in a 37°C cell culture incubator.Alternatives: Coverslips and culture wells can also be coated with 0.01% collagen (Collagen I, rat tail). When coating the coverslips and culture plates, dilute collagen in sterile water to 0.01% (w/v) and add 250 μL per well in a 24- well plate. Coat for 1–18 h in a 37°C cell culture incubator.
-
c.Before plating cells, pipette off gelatin solution and allow the coverslips or culture wells to dry for at least 2 h inside a tissue culture hood.
 CRITICAL: It is important to completely dry the plate before seeding the cells for better cell attachment.
CRITICAL: It is important to completely dry the plate before seeding the cells for better cell attachment. -
d.Re-suspend isolated type II cell pellet in media (HEPES-buffered DMEM, see ”Materials and equipment” for details). Plate 150,000–200,000 type II cells in each well, 500 μL per well.
-
a.
CRITICAL: In experiments with S1P treatment, add charcoal stripped FBS instead of regular FBS in cell culture medium.
Note: Some type II cell may die at 24–48 h after culture. To increase survival rate, it is important not to disturb the cells too much. Let cells sit for 48 h, then change medium.
-
5.Treat the cultured type II cells with S1P or JTE-013
-
a.Stock solution of S1P was made in ethanol at 1 mM and stored at −20°C.
-
b.Aliquot enough S1P solution needed for the experiment. Evaporate ethanol under inert gas, i.e., nitrogen or argon, in the tissue culture hood and reconstitute S1P to 125 μM in 4 mg/mL fatty acid free BSA made in sterile water.
 CRITICAL: The reconstituted S1P should be made fresh every time and apply to the cells immediately after made.Note: We recommend using inert gas to dry S1P to minimize oxidation. An alternative method is to use a speed vacuum centrifuge.
CRITICAL: The reconstituted S1P should be made fresh every time and apply to the cells immediately after made.Note: We recommend using inert gas to dry S1P to minimize oxidation. An alternative method is to use a speed vacuum centrifuge. -
c.Add S1P to cell culture medium containing charcoal stripped FBS to a final concentration of 1 μM.
 CRITICAL: Regular FBS contains high concentration of S1P. Make sure to use charcoal stripped FBS in experiments with S1P treatment.
CRITICAL: Regular FBS contains high concentration of S1P. Make sure to use charcoal stripped FBS in experiments with S1P treatment. -
d.Change half of the medium (250 μL per well) every day with freshly made S1P.Note: S1P turns over quickly and thus fresh S1P needs to be added to the culture every day. However, type II cells attach loosely in the first 2 days of culture. Therefore, caution should be taken to minimize the disturbance to the cells when changing the medium. We change only half of the medium, and when changing the medium, we keep the plate flat instead of tilted and add fresh medium slowly along the side of the culture wells.
-
e.To treat cells with JTE-013, dilute 20 mM JTE-013 (DMSO stock solution) in culture medium containing 10% regular FBS to a final concentration of 10 μM and add to the cells. Add same amount of DMSO to the control wells. Change medium after 2 days.
-
a.
-
6.Analyze the treated cells:
-
a.Fix cells with 4% PFA for 10 min at 20°C–25°C, remove PFA, and gently wash the cells with 1 mL PBS. Proceed for antibody staining for type I cell markers HopX and Ager (Yang et al., 2016) (Figure 4).
-
b.Or, lyse the cells in 1 mL Trizol and proceed for RNA extraction using a RNA extraction kit (Qiagen, https://www.qiagen.com/us/resources/resourcedetail?id=14e7cf6e-521a-4cf7-8cbc-bf9f6fa33e24&lang=en) and qRT-PCR to assess the expression of AT1 and AT2 marker genes, such as Sp-C for AT2 cells and HopX and Ager for AT1 cells (Chen et al., 2020).
-
a.
Figure 4.
2D culture of isolated type II cells
(A) At 24 h after culture initiation, most cells remained to be cuboidal shape.
(B) After cultured for 5 days, most cells acquired flattened type I cell-like shape.
(C) At 24 h culturing, most of the cells still express Sp-C.
(D and E) At 5 days culturing, many of the cells started to express type I cell marker HopX (D) or Ager (E).
Scale bar, 50 μm for (A) and (B), 70 μm for (C) and (D), 10 μm for (E).
3D co-culture of isolated type II cells with Mlg fibroblasts
Timing: 3 weeks
In this part, we will culture the isolated type II cells in a 3D culture system together with mouse lung fibroblasts Mlg on 24-well Transwell inserts. In this condition, type II cells will generate alveoli-like cysts consisting of both type I type I and type II cells and the progenitor properties of type II cells are maintained.
-
7.
Prepare for 3D culture: Grow Mlg and prepare Transwell insert. See above in “Prepare reagents for type II cell isolation and culture” for details
-
8.Culture isolated type II cells with Mlg fibroblasts in 3D inserts
-
a.Collect freshly isolated type II cells after either magnetic bead sorting or FACS sorting.
-
b.Pellet cells and re-suspend sorted type II cells at 100 cells/μL in 3D culture media (See “Materials and equipment” for details).
-
c.Trypsinize the cultured Mlg cells and re-suspend at 2,000 cells/μL in 3D culture mediaNote: Mlg cells harvested from one 75 mm flask at ~80% confluency are usually resuspended in ~1 mL medium.
-
d.Prepare Matrigel: thaw Matrigel on ice at 4°C for several hours.Note: We put 1 mL aliquots of Matrigel in a small styrofoam box with ice inside a 4°C refrigerator and let it thaw about 3–4 h before use.
-
e.In sterile 1.5 mL Eppendorf tubes, add
-
i.100 μL of the Matrigel +
-
ii.50 μL of Mlg (@100,000 cells/well) +
-
iii.50 μL of type II (@5,000 cells/well)
-
i.
-
f.Mix the cells and Matrigel well and plate the mixture (total 200 μL) in Transwell inserts
 CRITICAL: Matrigel starts to solidify above 10°C. Make sure to keep Matrigel, tubes, and cells on ice when making the mixture. Pre-chilling the tubes and pipette tips can be helpful.Note: Be gentle when mixing the cell and Matrigel and when plating the mixture to the Transwell to avoid bubbles.
CRITICAL: Matrigel starts to solidify above 10°C. Make sure to keep Matrigel, tubes, and cells on ice when making the mixture. Pre-chilling the tubes and pipette tips can be helpful.Note: Be gentle when mixing the cell and Matrigel and when plating the mixture to the Transwell to avoid bubbles. -
g.Incubate the plates for 30 min in a tissue culture incubator to solidify the Matrigel and then add 600 μL of warm 3D culture medium to the lower chamberNote: Do not add medium in the Transwell inserts. Alveolar organoids are grown in an air-liquid interface.
-
h.Grow cells in inserts for up to three weeks and change medium every other day.
-
i.Observe Tomato+ or GFP+ colonies in the Matrigel.
-
j.To fix cells in the Transwell inserts for further analysis, discard the culture medium and add 600 μL of 20°C–25°C 4% PFA to the wells. Cells are fixed at 20°C–25°C for 20 min and then further fixed at 4°C for 2 days (Figure 5).
-
k.After fixation, carefully peel off the membrane from the bottom of the Transwell insert and remove the Matrigel plug from the Transwell insert.Note: The Matrigel plug will still be soft after fixation, so be careful not to poke into the Matrigel when peeling off the membrane. We found that it is easier to remove the Matrigel plug by gently knocking the Transwell insert upside down and let the Matrigel plug to fall by itself.
-
l.To treat cells with JTE-013, add 10 μM JTE-013 to purified type II cells before mixing with Matrigel and to the 3D culture medium added to the wells. Change medium every other day with fresh JTE-013 added for 2–3 weeks.
-
a.
Alternatives: Primary PDGFRα+ fibroblast cells can be FACS isolated and used in 3D organoid culture instead of Mlg. Type II cells co-cultured with PDGFRα+ fibroblast give rise to more and larger organoids.
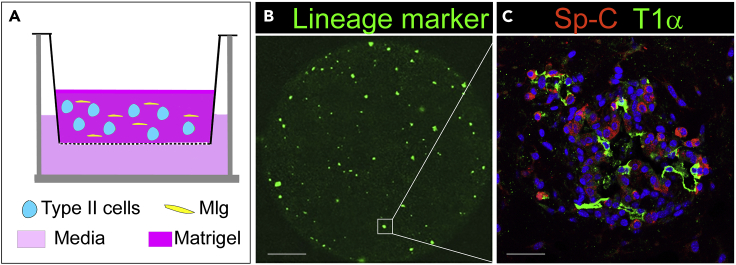
Figure 5.
3D co-culture of type II cells and Mlg
(A) Scheme of 3D co-culture of Mlg and type II cells using Transwell inserts.
(B) 2 weeks after culture, numerous colonies were formed as shown by GFP from the lineage tracing system of SpC-CreER/mTmG mice.
(C) The inserts were processed for paraffin embedding, sectioning, and antibody staining. One of the colonies was shown to be composed of Sp-C expressing type II cells and T1α expressing type I cells. Scale bar, 1 mm for (B), 30 μm for (C).
Expected outcomes
2D culture
Type II cells do not maintain as stem cells in 2D culture, and the purpose of the 2D culture is to study the type II cell differentiation process into type I cells. Type II cells remain to be cuboidal shape in the first day. After 48–72 h, the cells start to change into flat type I-like cell shape. At 24 h post culture, many cells still maintain their expression of type II cell marker Sp-C, but after 5 days, the cells start to express type I cell markers HopX and Ager (Figure 4). Co-expression of AT2 and AT1 markers are observed in cells during the transition. By day 7, almost all cells should have adopted the AT1 cell-like morphology and lost Sp-C expression.
If S1P is included in the medium, more of the type II cells can convert into type I cells. In contrast, if JTE-013 is included in the culture, type II to type I cell transition is disrupted (See Chen et al., 2020).
3D culture
Type II cell derived colonies can be visualized by the fluorescent lineage labeling (e.g., GFP for cells isolated from SpC-CreER/mTmG mice). Small colonies are usually formed approximately 1 week after culture and continue to expand in the following 1–2 weeks. 2–3 weeks after culture is a good timepoint to assess the formation of organoids, including number, size, cell composition, etc. The Matrigel plugs can be fixed, paraffin embedded, and stained for type II and type I cell markers. The colonies are alveoli-like cysts with some cells lining the lumen expressing type I cell marker T1α, and cells surround the lumen expressed type II cell marker Sp-C (Figure 5).
Different from 2D culture, some type II cells maintain the stem cell properties in 3D culture as they can be passaged. To passage the type II cells, organoids in Matrigel can be disassociated using dispase and type II cell can be FACS sorted based on their fluorescence signal and replated with fresh fibroblast cells (Mlg or PDGFRα+ fibroblast cells), to grow in to new organoids (Barkauskas et al., 2013).
If JTE-013 is included in the culture, there will be less type I cells in the alveoli-like cysts (See Chen et al., 2020)
Limitations
The above discussed type II cell yield and purity is based on isolation performed on young (8–12 weeks) and uninjured mice. We typically get over 85% pure type II cells after negative selection using the CD32/CD45 plates, as assessed by SP-C staining. Both aging and lung injury could reduce the purity at this step. Therefore, it is important to use beads sorting or through FACS, to further purify the cells.
In 2D culture, a lot of cells would be dead after the first 1–2 days of culture.
Troubleshooting
Problem 1
Low survival rate of 2D culture (step 4).
Potential solution
For 2D culture, to increase survival rate, it is important not to disturb cells too much. Let cells sit for 48 h, then change media. Alternatively, the culture dish or glass slips used for growing type II cells can be coated with collagen at 0.01% (w/v) or coated with a thin layer of 50% Matrigel. However, with Matrigel coating, the cells sometimes did not attach to the coverslips and were washed away while proceed with antibody staining.
Problem 2
For 2D culture, sometimes when the culture condition is sub-optimal, the WT control type II cells remain to be cuboidal shape after 4–5 days of culture (Morphology remain similar to Figure 4A even after 4–5 days culture) (step 4).
Potential solution
If this happens, the culture needs to be started over with fresh medium.
Problem 3
Type II cells do not attach well on gelatin coated coverslips (step 4).
Potential solution
Thoroughly wash coverslips by heating the coverslips in 1 N HCl at 50°C–60°C for 4–16 h for a better coating and coat coverslips with 0.01% collagen (w/v) instead of gelatin.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yuru Liu, yuruliu@uic.edu.
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate datasets/code.
Acknowledgments
This work was supported by grants from NHLBI R01 HL105947 (Y.L.), AHA17GRNT33700246 (Y.L.), and AHA19POST34380566 (Q.C.).
Author contributions
Conceptualization, Y.L.; Methodology, Q.C. and Y.L.; Investigation, Q.C. and Y.L.; Writing, Q.C. and Y.L., Funding Acquisition, Y.L. and Q.C., Supervision, Y.L.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100241.
Contributor Information
Qian Chen, Email: qianc15@uic.edu.
Yuru Liu, Email: yuruliu@uic.edu.
References
- Barkauskas C.E., Michael J., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Rehman J., Chan M., Fu P., Dudek S.M., Natarajan V., Malik A.B., Liu Y. Angiocrine sphingosine-1-phosphate activation of S1PR2-YAP signaling axis in alveolar type II cells is essential for lung repair. Cell Rep. 2020;31:107828. doi: 10.1016/j.celrep.2020.107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs L.G. Isolation and culture of alveolar type II cells. Am. J. Physiol. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- Kalina M., Mason R.J., Shannon J.M. Surfactant protein C is expressed in alveolar type II cells but not in Clara cells of rat lung. Am. J. Respir. Cell Mol. Biol. 1992;6:594–600. doi: 10.1165/ajrcmb/6.6.594. [DOI] [PubMed] [Google Scholar]
- Korfhagen T.R., Glasser S.W., Wert S.E., Bruno M.D., Daugherty C.C., McNeish J.D., Stock J.L., Potter S.S., Whitsett J.A. Cis-acting sequences from a human surfactant protein gene confer pulmonary-specific gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA. 1990;87:6122–6126. doi: 10.1073/pnas.87.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Bhang D.H., Beede A., Huang T.L., Stripp B.R., Bloch K.D., Wagers A.J., Tseng Y.H., Ryeom S., Kim C.F. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kumar V.S., Zhang W., Rehman J., Malik A.B. Activation of type II cells into regenerative stem cell antigen-1(+) cells during alveolar repair. Am. J. Respir. Cell Mol. Biol. 2015;53:113–124. doi: 10.1165/rcmb.2013-0497OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J.R., Barkauskas C.E., Cronce M.J., Xue Y., Harris J.R., Liang J., Noble P.W., Hogan B.L. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hernandez B.J., Martinez Alanis D., Narvaez del Pilar O., Vila-Ellis L., Akiyama H., Evans S.E., Ostrin E.J., Chen J. The development and plasticity of alveolar type 1 cells. Development. 2016;143:54–65. doi: 10.1242/dev.130005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets/code.