Figure 4.
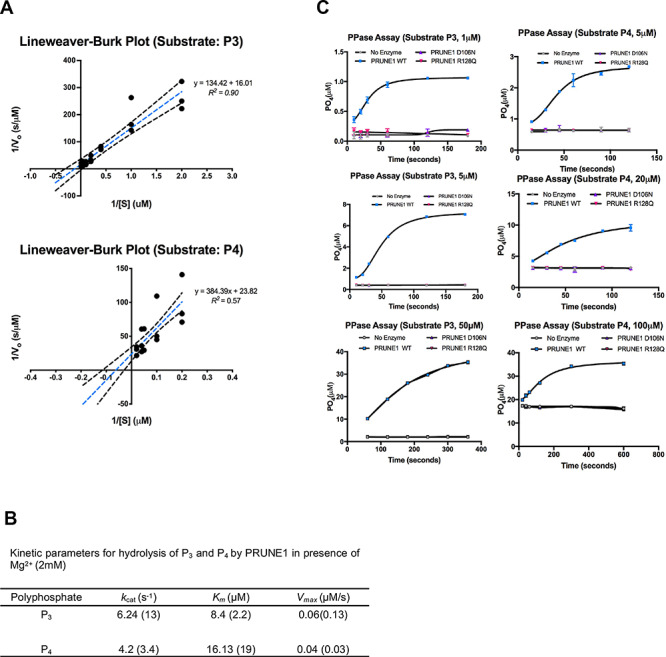
D106N and R128Q variants result in loss of short chain exopolyphosphatase activity. (A) Lineweaver−Burk plots depicting short-chain exopolyphosphatase kinetics of wild-type PRUNE1 using sodium tripolyphosphate (P3) and sodium tetrapolyphosphate (P4) as substrates. Dotted black lines represent 95% CI. (B) Kinetic parameters for hydrolysis of polyphosphates (P3 and P4) by wild-type PRUNE1 in the presence of Mg2+ (2 mM) as the cofactor. Values reported by Tammenkoski et al. are shown within parenthesis (17). (C) Short-chain exopolyphosphatase activity of wild-type, D106N and R128Q mutants on P3 and P4 determined using fixed-time BIOMOL Green phosphate detection assay. Data represented as mean ± SEM over three independent experiments with six technical replicates per sample.
