Abstract
Listeria monocytogenes is a facultative intracellular pathogen responsible for the life-threatening disease listeriosis. The pore-forming toxin listeriolysin O (LLO) is a critical virulence factor that plays a major role in the L. monocytogenes intracellular lifecycle and is indispensable for pathogenesis. LLO is also a dominant antigen for T cells involved in sterilizing immunity and it was proposed that LLO acts as a T cell adjuvant. In this work, we generated a novel full-length LLO toxoid (LLOT) in which the cholesterol-recognition motif, a threonine-leucine pair located at the tip of the LLO C-terminal domain, was substituted with two glycine residues. We showed that LLOT lost its ability to bind cholesterol and to form pores. Importantly, LLOT retained binding to the surface of epithelial cells and macrophages, suggesting that it could efficiently be captured by antigen-presenting cells. We then determined if LLOT can be used as an antigen and adjuvant to protect mice from L. monocytogenes infection. Mice were immunized with LLOT alone or together with cholera toxin or Alum as adjuvants. We found that mice immunized with LLOT alone or in combination with the Th2-inducing adjuvant Alum were not protected against L. monocytogenes. On the other hand, mice immunized with LLOT along with the experimental adjuvant cholera toxin, were protected against L. monocytogenes, as evidenced by a significant decrease in bacterial burden in the liver and spleen three days post-infection. This immunization regimen elicited mixed Th1, Th2, and Th17 responses, as well as the generation of LLO-neutralizing antibodies. Further, we identified T cells as being required for immunization-induced reductions in bacterial burden, whereas B cells were dispensable in our model of non-pregnant young mice. Overall, this work establishes that LLOT is a promising vaccine antigen for the induction of protective immunity against L. monocytogenes by subunit vaccines containing Th1-driving adjuvants.
Keywords: Listeria monocytogenes, listeriolyin O, cholesterol-dependent cytolysin, vaccine, cancer immunotherapy
Introduction
Listeria monocytogenes is a foodborne pathogen and the causative agent of the life-threatening disease listeriosis. The risk and severity of listeriosis are significantly increased among the elderly, pregnant women, infants, and individuals with a compromised immune system1. Clinical manifestations of listeriosis include septicemia, meningitis, encephalitis, miscarriage, stillbirth, and severe infection of neonates1–6, with an associated fatality rate of 16–25% despite treatment7. Although the food industry has rigorous standards for prevention and surveillance of L. monocytogenes contamination, the reported incidence of listeriosis has not significantly decreased since 20108. Without reductions in the incidence of listeriosis and its associated high fatality rate, a vaccine targeting L. monocytogenes could offer an effective preventative measure to reduce the risk of this deadly disease in susceptible populations. In particular, the aging population, representing approximately 80% of listeriosis patients, is constantly increasing worldwide8. In addition, a vaccine for animal use could protect livestock and decrease contamination of related food products9–11.
The pore-forming toxin LLO is required for host cell invasion and pathogenesis as LLO-deficient L. monocytogenes strains are avirulent12. LLO forms pores across host cell membranes leading to ionic fluxes, activation of signaling pathways, and mitochondrial remodeling, which facilitate L. monocytogenes internalization, vacuolar escape, cell-to-cell spread, and intracellular proliferation13–17. In addition to its role as a virulence factor, LLO is a major source of CD4+ and CD8+ T cell epitopes during the adaptive immune response to L. monocytogenes in mice18,19. L. monocytogenes and LLO display immune stimulatory activities that raised considerable interest in cancer immunotherapy20–26. Indeed, native LLO, LLO variants that are less hemolytic, and truncated LLO variants were proposed to stimulate antigen-specific T cell responses directed against cancer antigens22,24,25. Live-attenuated L. monocytogenes strains have shown promise in providing protection against both infections and cancer in experimental animal models27–32 and several of these vaccines are in clinical development22,33–36. However, there are reported dangers in utilizing L. monocytogenes live-attenuated strains in immunocompromised individuals37. Given that populations at higher risk for listeriosis and cancer patients may have weakened or altered immunity, a subunit vaccine would be a safer alternative. As such, subunit vaccines that utilize major L. monocytogenes virulence factors, including LLO, GAPDH peptides, and p60 have been developed38–41. Most of these vaccines induce potent T cell responses (CD4+ and CD8+), which are essential for the acquisition of sterilizing immunity against L. monocytogenes38–41 and play critical roles in anti-cancer immunity20,24.
In this study, we generated a novel LLO toxoid (LLOT) devoid of hemolytic activity. This was achieved by substituting the threonine 515-leucine 516 pair, which is critically involved in cholesterol recognition and pore formation, with glycine residues42. We characterized the host cell binding and pore-forming abilities of LLOT. We then established if LLOT could act as a vaccine antigen and adjuvant alone or in combination with cholera toxin and Alum adjuvants to protect against L. monocytogenes infection using the systemic murine infection model of listeriosis.
Materials and Methods
Generation of LLO variants and LLO toxoid
The gene coding for six-His-tagged LLOT with the substitutions T515G and L516G was generated by PCR-based site-directed mutagenesis using the pET29b plasmid harboring wild type (WT) hly (the gene coding LLO) as a template and the Forward – 5’-gaa ata tct cca tct ggg gca ccg ggg gtt atc cga aat ata gta ata aag-3’ and Reverse – 5’-ctt tat tac tat att tcg gat aac ccc cgg tgc ccc aga tgg aga tat ttc-3’ mutagenic primers as described previously14. The gene coding for six-His-tagged LLOW492A was generated using the same strategy and the Forward – 5’-ggt tta gct tgg gaa tgg gcg aga acg gta att gat gac cgg-3’ and Reverse – 5’-ccg gtc atc aat tac cgt tct cgc cca ttc cca agc taa acc-3’ primers43. The gene coding for the six-His-tagged truncated listeriolysin O LLO (LLO D1–3) was amplified by PCR from the WT sequence of hly using the Forward – 5’-aac gtg cat atg gat gca tct gca ttc aat aaa G-3’ and Reverse – 5’-att ctc gag tgt ata agc ttt tga agt tgt-3’44 primers and cloned into pET29b using NdeI and XhoI restriction sites. Purification of recombinant LLO variants was performed as previously described14,45. Endotoxin measurements were performed using the Chromogenic Endotoxin Quant Kit (Pierce), and LLOT was inoculated at 200 μg/ml with endotoxin levels strictly below 36 EU/ml46. For analysis of LLO by SDS-PAGE, 1 μg of toxin was loaded, and gels were stained with Coomassie blue and imaged using a ChemiDoc XRS imaging system (Bio-Rad).
Circular Dichroism spectroscopy
A Jasco J-815 Circular Dichroism spectrometer was used to acquire LLO and LLOT spectra at 10°C with a 1 mm cuvette at a protein concentration of 0.5 mg/ml in 20 mM sodium phosphate buffer at pH 6. Spectra were recorded at 1 nm wavelength intervals (190 to 255 nm). The spectra are the average of three scans.
Cholesterol Binding Assay
Spots (2 μl) of a serially diluted ethanol-cholesterol solution were deposited onto a PVDF membrane and air-dried. The membranes were saturated by incubation in 20 mM Tris buffer saline (TBS) containing 4% nonfat milk and 0.2% Tween 20 at pH 7.4. LLO and LLOT (20 μg/ml) were incubated at 4° for 3 h in TBS with 0.2% Tween 20. After washes, rabbit anti-LLO Abs (Abcam) were incubated for 1 h in TBS with 0.1% Tween 20, followed by washes and incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies in TBS with 0.1% Tween 20. LLO was detected with ECL Western Blotting Detection Kit (Amersham). We verified by immunoblotting that the rabbit anti-LLO antibodies recognize LLO and LLOT with similar efficiency (data not shown).
LLO binding assays and HepG2 invasion assays
HeLa cells (1.5 × 105 /well) were grown for 24 h in 6-well plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (HIFBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were incubated for 30 min in FBS-free medium +/− 5 mM methyl-β-cyclodextrin (mβCD) at 37°C to deplete cholesterol. Cells were incubated for 10 min in FBS-free medium with LLO, LLOT, or LLO D1–3 at 1, 2, or 5 nM concentrations at 4°C. Cells were then washed with cold PBS and lysed with lysis buffer (150 mM NaCl, 20 mM Tris/HCl, 2 mM EDTA, 1% NP-40, and protease inhibitor cocktail (Roche)). Cell lysates were subjected to immunoblot analysis using anti-LLO (Rabbit polyclonal from Abcam) or anti-actin antibodies (Cell Signaling) and secondary antibodies conjugated to HRP (Cell Signaling). Detection was performed using Amersham ECL Select Reagent Kit (GE Healthcare) and a ChemiDoc XRS Imaging System (Bio-Rad). THP-1 cells were cultured in RPMI-1640 supplemented with 10% HIFBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). 2 × 106 cells were washed with FBS-free medium and incubated with 1 nM and 5 nM LLO or LLOT for 10 min at 4°C. THP-1 cells were washed with PBS, lysed, and subjected to immunoblot analysis as described above. For invasion assays, HepG2 cells were cultured in 24-well plates and incubated with bacteria at multiplicity of infection (MOI) 5 for 30 min in the presence or absence of LLO-neutralizing antibodies. Cells were washed, fixed and labeled as previously described to measure the percentage of bacterial internalization31. All human cell lines used in this study were authenticated by ATCC.
Hemolysis Assays
Human blood was drawn in heparinized tubes from healthy adult volunteers with approval of the Ohio State University Review Board. Erythrocytes were isolated after centrifugation of blood on Polymorphprep (Axis-Shield, Oslo, Norway) and were washed with Alsever’s solution. Erythrocytes were washed 3 times with PBS and diluted to at 4 × 107 cells/ml. Duplicate serial dilutions of native LLO, LLOW492A, LLOD1–3, and LLOT were made at 4°C in a round bottom 96-well plate, and 160 μl of cold erythrocytes suspension were added in each well. Concentrations tested were: native LLO (100 nM – 0.1 nM), LLOW492A (3,000 nM – 1.5 nM), LLOT (10,000 nM – 5 nM), LLOD1–3 (6,000 nM – 3 nM). Plates were incubated for 30 min at 37°C, centrifuged, and the supernatants were transferred to a flat bottom 96-well plate for reading their absorbance at 540 nm. Erythrocytes were treated with 0.1% Triton X-100 (100% hemolysis) and with PBS as positive and negative controls, respectively. The concentration of toxin leading to 50% hemolysis (EC50) was determined by polynomial regression using Graph Pad Prism 7 software (GraphPad Software Inc, La Jolla, CA).
Immunization and T cell depletion
All animal protocols were approved by the Ohio State University Laboratory Animal Care and Use Committee. Seven to eight week-old WT C57BL/6 or C57BL/6-Igh-6tm1Cgn (B cell-deficient, μMT−/−)47 mice, from The Jackson Laboratory (Bar Harbor, ME), were housed in the vivarium for one week before starting immunization. Mice were immunized on days 0, 7, and 14 by intraperitoneal injection of 100 μl of injectable grade PBS containing: PBS only (control groups), 20 μg LLOT in the presence or in the absence of 1 μg cholera toxin (List Biological Laboratories, Inc, Campbell, CA), or 20 μg LLOT adsorbed on 40 μg Imject Alum (a mixture of aluminum hydroxide and magnesium hydroxide; ThermoFisher Scientific). LLOT was adsorbed to Alum via gentle mixing for 45 min at 4°C. For T cell depletions, mice were treated with 300 μg of rat anti-mouse CD4 monoclonal antibody (mAb) (clone GK1.5, BioXcell) plus 300 μg rat anti-mouse CD8α mAb (clone 53–6.7, BioXcell) by intraperitoneal injection 48 h before infection. Mice received a second injection of 100 μg of anti-T cell antibodies 24 h post-infection (200 μg total). Control mice received intraperitoneal injection of rat IgG2a and IgG2b isotype controls (BioXcell) at the same doses. Blood was collected via submandibular cheek bleed on days 14, 21, and 28, or via cardiac puncture after infection and sacrifice. Serum was obtained by centrifugation of the clotted blood (1,500 x g for 15 min at 4°C). For monitoring T cell depletion, blood was collected into heparinized tubes and diluted 1:1 with PBS. Red blood cells were lysed by addition of 0.84% ammonium chloride buffer. After 5 min incubation on ice, the samples were centrifuged at 1500 rpm for 5 min at 4°C. Supernatant was discarded and samples were washed twice with FACS buffer before staining with the following fluorescent Abs: FITC anti-CD11b (clone M1/70, BioLegend), PerCP/Cy5.5 anti-CD8a (clone 53–6.7, BioLegend), APC anti-CD11c (clone N418, eBiosciences), Alexa Fluor 700 CD3 (clone 17A2, BioLegend), APC/Fire 750 CD19 (clone 6D5, BioLegend), Brilliant Violet 421 F4/80 (clone BM8, BioLegend), PE NK1.1 (clone PK136, BD Biosciences), PE-Cy7 CD4 (clone RM4–5, BioLegend). Cells were fixed with paraformaldehyde and analysis was performed using Attune Nxt Acoustic Focusing Cytometer (Thermo Fisher). For IgG isolation, larger volumes of blood were collected by cardiac puncture immediately after sacrifice of the animals. IgG were isolated using protein G coupled agarose beads according to the manufacturer’s instructions (Pierce).
Bacterial Cell Culture and Mouse Infection
WT and LLO-deficient L. monocytogenes (isogenic strains DP10403S and DP-L2161) were grown overnight at 37°C in brain heart infusion (BHI). For cell or mice infections, bacteria were diluted 1/20 in BHI and grown at 37°C until OD600 = 0.7–0.8. Bacteria were washed three times and diluted in injectable grade PBS (for animal infections) or in cell culture medium (for cell invasion). Mice were inoculated by tail vein injection with L. monocytogenes (2 × 104 in 100 μl injectable grade PBS) on day 28. After 72 h, mice were euthanized and livers, spleens, and blood were collected. Organs were homogenized in PBS. Homogenates were serially diluted, plated on BHI agar plates, and incubated at 37°C for 48 h to determine the colony forming units (CFUs).
Evaluation of LLO-specific antibody titers by ELISA
Briefly, 100 μl of LLOT (5 μg/ml in PBS) were added to ELISA plates and incubated at 4°C overnight. Plates were washed with cold PBS and blocked for 2 h with PBS 1% BSA. Plates were washed, and 100 μl of PBS 1% BSA containing serial dilutions of sera were added. After overnight incubation at 4°C, the LLOT-specific antibodies were detected with HRP-conjugated anti-mouse IgG sera (1:3000 dilution) (Southern Biotech Associates Inc.). Alternatively, to measure IgG subclasses, biotin-conjugated rat anti-mouse IgG1, IgG2a/c, IgG2b, or IgG3 monoclonal Abs and HRP-conjugated streptavidin (BD Biosciences) were used (0.5 μg/ml). The HRP substrate ABTS (2, 2’-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt, Sigma-Aldrich) was added, and the antibody titers were determined based on the last dilution of samples with an absorbance of >0.1 above that of samples from control mice mock immunized with PBS.
Evaluation of the production of LLO-neutralizing antibodies
To test for LLO neutralization, a kinetic hemolytic assay was performed. IgG were purified from serum collected from immunized mice using protein G-agarose (Pierce) according to the manufacturer’s instructions. LLO and LLOT (5 nM in PBS) and various dilutions of purified serum IgG were pre-incubated on ice in a 96-well plate for 15 min before the addition of erythrocytes at 4 × 107 cells/ml, to test LLO activity. Triton X-100 (0.1%) and PBS served as positive and negative controls for hemolysis, respectively. Samples were transferred to a spectrophotometer at 37°C and the absorbance (700 nm) was measured at 60 sec intervals for 30 min.
Analysis of LLOT-specific T helper cell cytokines responses
Spleens (2 spleens per experimental condition for control and LLOT and 3 spleens per experimental condition for all other groups) were aseptically removed from mice 38 days after initial immunization and homogenized by pressing through a cell strainer. Red blood cells were lysed by incubation in 0.84% ammonium chloride, and, following a series of washes in RPMI 1640, spleen cells were suspended in RPMI 1640 supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum. 100 μl splenocytes (5 × 106 cells/ml) were added to each well (3 wells per spleen) of a 96-well micro-titer plate and cultured either alone, or in the presence of 5 μg/ml LLOT, for 5 days at 37 °C and 5% CO2. Cells were stimulated with PMA and Ionomycin (BDPharmangen) and incubated for 1 h at 37 °C. Cytokine secretion was blocked by adding the protein transport inhibitor Golgistop (BD-Pharmangen), and cells were incubated at 37 °C for 5 h. Cells were then collected and washed twice with FACS buffer (PBS, 2% BSA, 0.01% NaN3). For identifying T-cells, cells were labeled with Alexa Fluor 700 anti-CD3 (clone 17A2) and Alexa Fluor 750 anti-CD4 (clone GK1.5) monoclonal antibodies (Biolegend) for 30 min at 4°C, then washed twice with FACS buffer (BD-Pharmagen). For intracellular cytokine staining, cells were incubated with Fixation-Permeabilization Buffer (BD-Pharmagen) for 20 min at 4°C and washed twice with the permeabilization buffer (BD-Pharmangen). Cells were then labeled with cytokine-specific antibodies [Alexa Fluor 488-IFNγ (clone XMG1.2), PerCP Cy5.5-TNFα (clone MP6-XT22), PE-IL-5 (clone TRFK5), Alexa Fluor 647-IL-21 (clone IC594R, R &D System), PECy7 IL-10 (clone JES5–16E3), Brilliant Violet 650 IL-17 (clone TC11–18H10.1), Brilliant Violet 605 IL-4 (clone 11B11)] for 30 min at 4°C. Cells were washed twice with the permeabilization buffer and then twice with the FACS buffer. Cells were suspended in FACS buffer and analyzed with an Attune NxT flow cytometer (Thermo Fisher Scientific). Data were analyzed by triple gating (CD3+CD4+Cytokines+). Statistical analyses were performed by one-way ANOVA using Graph Pad Prism7, and significant differences were considered at p ≤ 0.05 (*).
Results
Generation of a full-length non-hemolytic listeriolysin O toxoid (LLOT)
LLO belongs to the cholesterol-dependent cytolysins (CDCs) family of bacterial toxins, which form 30 to 50 nm diameter homo-oligomeric pores in cholesterol-rich membranes48. CDC binding to cholesterol is indispensable for pore formation, and the cholesterol recognition motif was identified as a conserved threonine-leucine pair located in their C-terminal membrane binding domain (Domain 4)42. We generated a full-length LLO toxoid, referred to as LLOT, by substitution of the cholesterol-binding threonine-leucine pair with glycine residues (T515G/L516G)42. We compared the properties of LLOT to those of native LLO, a truncated LLO variant devoid of domain 4 (LLO D1–3), and a LLO variant with the amino acid substitution W492A in domain 4 that was previously reported as an attenuated LLO variant (LLO W492A)43. Recombinant 6-his-LLO, -LLOT, -LLO D1–3, and -LLO W492A were characterized by SDS-PAGE (Fig. 1A) and by circular dichroism (Fig. 1B) to compare LLO and LLOT secondary and tertiary structures49. The spectra for LLOT and LLO were similar, indicating that the toxoid is properly folded (Fig. 1B). A dot blot assay confirmed that, unlike LLO, LLOT was unable to bind cholesterol, as expected (Fig. 1C). We also tested if LLOT could bind host cell membranes, since most CDCs such as PFO, PLY, and SLO are thought to lose binding to host cell membranes in the absence of the cholesterol-recognition motif42. Unlike these other CDCs, LLOT retained binding to HeLa (human epithelial cell line) and THP-1 (human monocyte cell line) cells, though not to the same extent as native LLO. A concentration of 2 nM LLOT provided equivalent binding to HeLa cells as 1 nM LLO and 5 nM LLOT were necessary to achieve similar binding to THP-1 cells as 1 nM LLO (Figs. 2A & 2D). Importantly, LLO D1–3 did not bind host cells, confirming that only domain 4 mediates binding to host cells (Fig. 2C). LLO and LLOT binding to cholesterol-depleted cells was reduced, but not abrogated (Fig. 2B). Together, these results indicate that cholesterol is not the only host cell ligand for LLO. Furthermore, the fact that LLOT binding to cholesterol-depleted cells is reduced, also strongly suggest that cholesterol indirectly affects toxin binding to host cells, for example, by affecting the biophysical properties of the membrane and/or access of LLO to other membrane ligands. Finally, the hemolytic activity of LLOT was markedly decreased by 3,000-fold and 60-fold compared with native LLO and LLO W492A, respectively. As expected, LLOT hemolytic activity was nearly as low as the truncated LLO D1–3 variant, which is unable to bind host cells (Fig. 3). These results confirm the indispensable role of the threonine 515-leucine 516 pair in LLO binding to cholesterol and pore formation. In conclusion, LLOT is a full-length LLO variant that retains its antigenic peptides, folds properly, and binds host cells, including antigen-presenting cells, which are critical features for efficient capture and presentation by antigen-presenting cells.
Figure 1. LLOT does not bind to cholesterol.
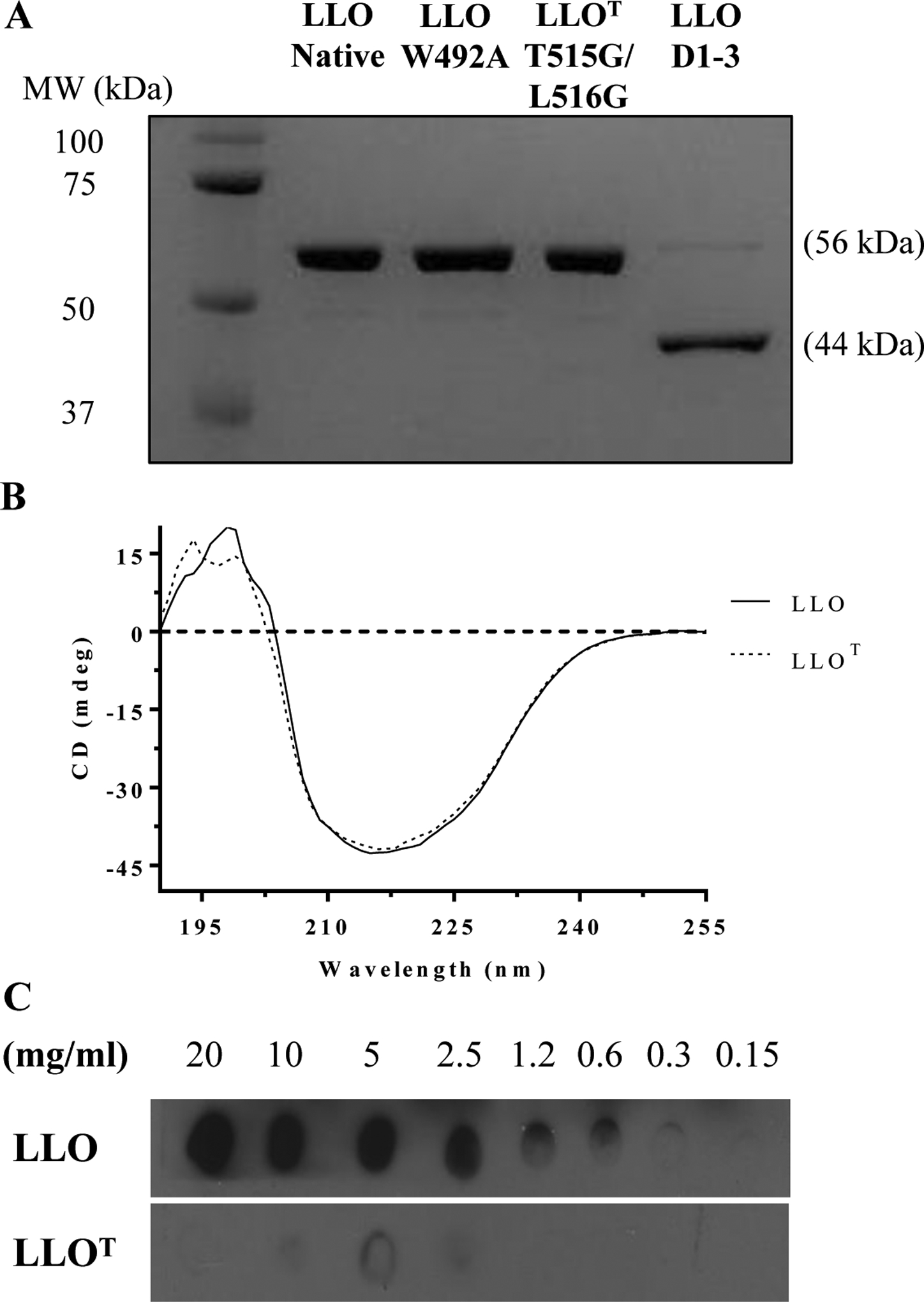
(A) Recombinant LLO, LLOT, LLO W492A, and LLO D1–3 (1 μg) were subjected to SDS-PAGE and stained with Coomassie blue. (B) Representative CD spectra of LLO and LLOT (0.5 mg/ml). (C) LLO and LLOT were incubated on a PVDF membrane pre-coated with a serial dilution of an ethanol-cholesterol solution. Pre-coated membranes were then incubated with LLO or LLOT, and binding to cholesterol was visualized by immunoblot analysis using anti-LLO antibodies.
Figure 2. LLOT binds to host cells.
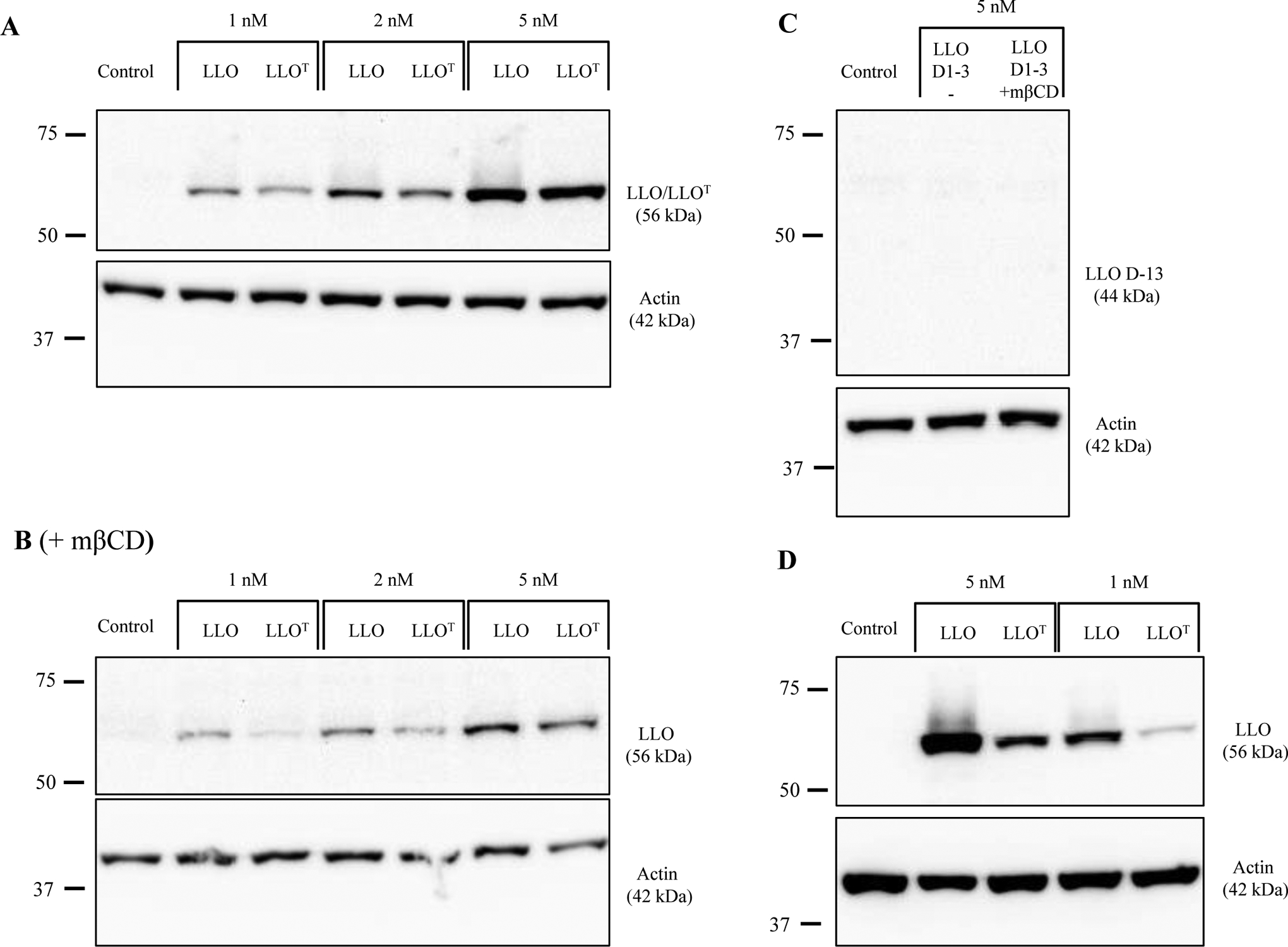
Control HeLa cells (A), and HeLa cells pre-treated with 5 mM methly-β-cyclodextrin (mβCD) (B), were incubated 10 min at 4°C with LLO or LLOT. (C) HeLa cells pre-treated, or not, with 5 mM mβCD were incubated 10 min at 4°C with LLO D1–3. (D) THP-1 cells were incubated 10 min at 4°C with LLO or LLOT. (A, B, C, D) After incubation at 4°C, cells were washed and lysed at 4°C, and cell lysates were analyzed by immunoblotting using anti-LLO and anti-actin primary antibodies. Representative immunoblots were selected from at least 3 independent experiments.
Figure 3: LLOT is non-hemolytic.
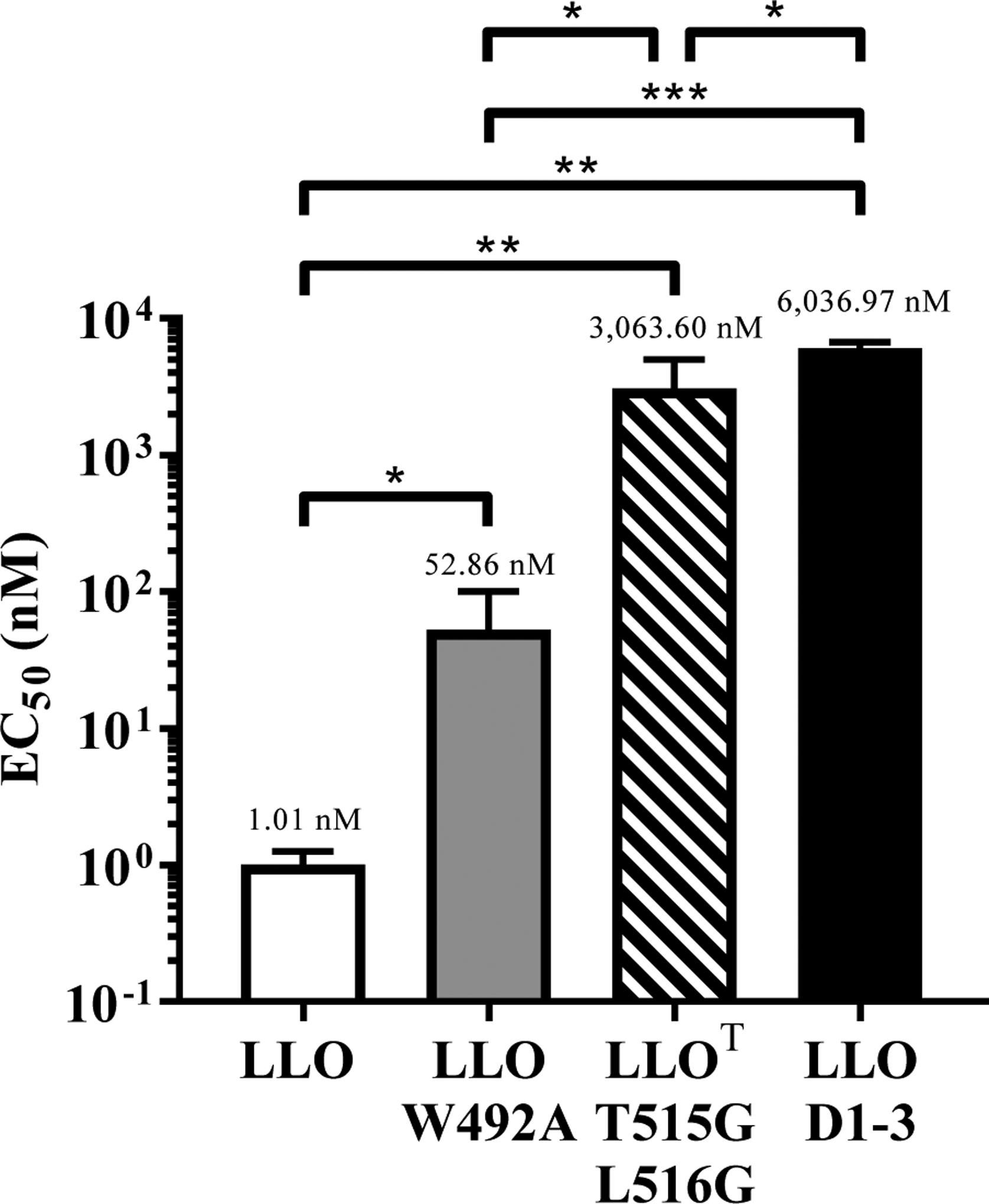
Hemolytic activity of LLO, LLO W492A, LLOT, and LLO D1–3 was measured from three independent experiments, each performed in duplicate, as EC50, i.e. the toxin/toxoid concentration required to cause 50% hemolysis. Average EC50 is shown above each column. P values were calculated using a two-tailed Student’s t-test (* = P, <0.05; ** = P <0.01; *** = P <0.001).
Immunization with LLOT plus cholera toxin, but not Alum, protects mice against L. monocytogenes
Sterilizing immunity against L. monocytogenes requires CD4+ and CD8+ T cells29,50. In addition, passive transfer of monoclonal LLO-neutralizing antibodies efficiently protected naïve mice against sub-lethal and lethal doses of L. monocytogenes51. This led us to hypothesize that a vaccine that elicits the production of LLO-neutralizing antibodies and T cell immunity could offer extensive protection against L. monocytogenes. We determined if LLOT administered alone, or together with the experimental adjuvant cholera toxin (CT), could promote immunization of mice against L. monocytogenes. Cholera toxin was used for its broad effects on T and B cell stimulation52. Mice were treated with PBS, LLOT, CT, or LLOT + CT via intraperitoneal injections at weekly intervals for 3 weeks. At day 28, mice were challenged with 2 × 104 L. monocytogenes by tail vein injection. Bacterial burden was measured in the spleen and liver three days post-infection. As shown in Figure 4, mice immunized with LLOT plus CT displayed significant reductions in bacteria in the spleen and liver when compared to the groups that received LLOT alone, CT alone, or PBS.
Figure 4. Immunization with LLOT plus cholera toxin protects mice against infection by L. monocytogenes.
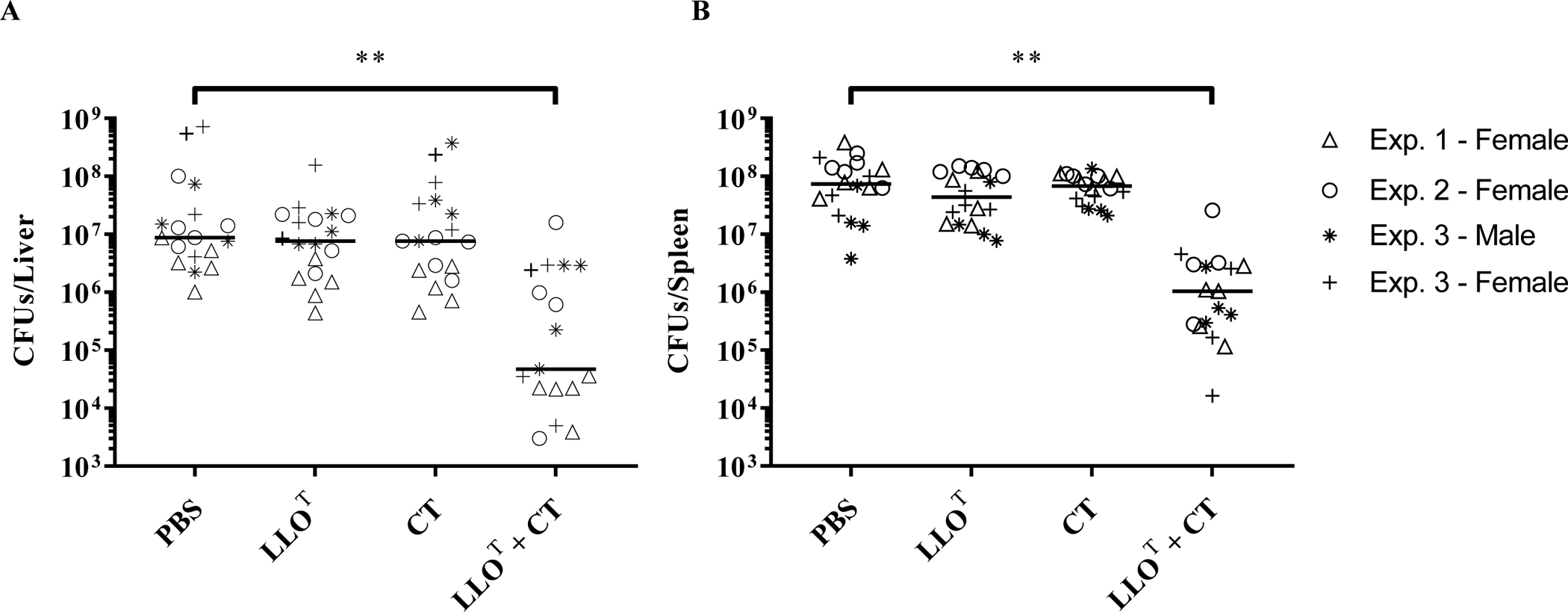
Mice were immunized at weekly intervals, for 3 consecutive weeks, by intraperitoneal injection of PBS (negative control), cholera toxin adjuvant (CT, 1 μg), LLOT (20 μg), or LLOT (20 μg) plus cholera toxin (1 μg) (LLOT + CT). On day 28, mice were intravenously inoculated with 2 × 104 L. monocytogenes and sacrificed after 72 h to collect organs and enumerate bacterial colony forming units (CFUs) in the liver (A) and spleen (B). Results are expressed as CFUs/organ and medians are presented. Data are from 3 independent experiments with 5 females per condition in experiment 1, 5 females per condition in experiment 2, and 4 males and 4 females per condition in experiment 3. Statistical significance was calculated using a two-sided Mann-Whitney test, ** P < 0.01.
To examine the possibility that production of anti-LLO neutralizing antibodies could play a role in the protection of mice, as was observed with the injection of a monoclonal anti-LLO neutralizing antibody51, we also tested the efficacy of the Alum adjuvant. Alum is a widely used vaccine adjuvant that predominantly induces strong Th2-mediated antibody responses to antigens53. However, mice immunized with LLOT plus Alum were not protected against L. monocytogenes (Fig. 5). We used male and female mice throughout these studies and did not observe any sex-specific response to vaccination.
Figure 5. Immunization with LLOT plus Alum does not protect mice against infection by L. monocytogenes.
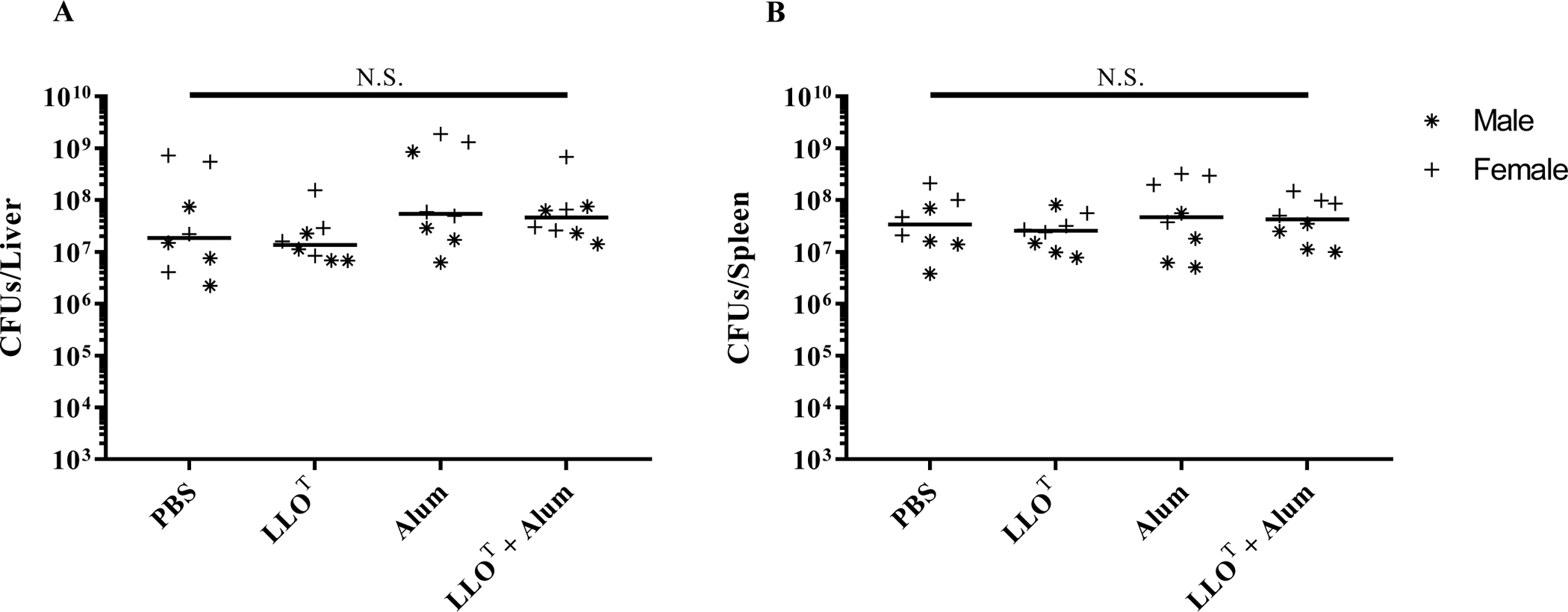
Mice were immunized at weekly intervals, for 3 consecutive weeks, by intraperitoneal injection of PBS (negative control), LLOT (20 μg), Alum (40 μg), or LLOT (20 μg) plus Alum (40 μg). At day 28, mice were intravenously inoculated with 2 × 104 L. monocytogenes and sacrificed after 72 h to collect organs and enumerate bacterial colony forming units (CFUs) in the liver (A) and spleen (B). Data are from one experiment, including 4 males plus 4 females for each experimental condition. Results are expressed as CFUs per organ and medians are presented. Statistical analysis was made using a two-sided Mann-Whitney test, N.S. = Not statistically significant.
Immunization with LLOT plus cholera toxin, but not Alum, leads to significant production of LLO-neutralizing antibodies and diverse antibody isotype response
To address the difference in the ability of CT and Alum adjuvants to elicit protective immunity, we next measured the anti-LLOT IgG titers in the various groups of animals. Mice immunized with LLOT alone or together with adjuvants produced anti-LLOT IgG1, IgG2a, IgG2b, and IgG3 (Fig. 6). Notably, CT significantly enhanced the production of all measured isotypes of LLOT-specific antibodies compared to LLOT alone, whereas Alum only significantly increased the production of IgG1. Indeed, LLOT alone or LLOT + Alum led to similar levels of anti-LLO IgG2a, IgG2b, and IgG3. Mice immunized with LLOT + CT produced more IgG1 than IgG2a, a profile previously seen when CT was administered with inert antigens such as ovalbumin54. Finally, IgGs purified from mice treated with LLOT + CT efficiently neutralized LLO activity, which was not observed with IgGs purified from mice treated with LLOT + Alum (Fig. 7). Together, these data show that, unlike Alum, CT induced efficient production of anti-LLO IgG2a/b and IgG3 isotypes and neutralizing anti-LLO antibodies. To determine if these anti-LLO antibodies could reduce bacterial burden in naïve mice, we performed adoptive transfer of 100 μl serum collected from LLOT + CT immunized mice, and from non-immunized control mice, into naïve mice by intraperitoneal injection and subsequently infected these mice with L. monocytogenes. However, serum transfer had no observable effect on infection (data not shown). Our concern with this experimental approach was that transfer of a small volume of serum could lead to unconvincing results if high antibody titers were necessary for protection. This prompted us to assess the effect of immunization in B-cell deficient mice as an alternative approach.
Figure 6. LLOT-specific IgG production in mice immunized with LLOT and adjuvants.
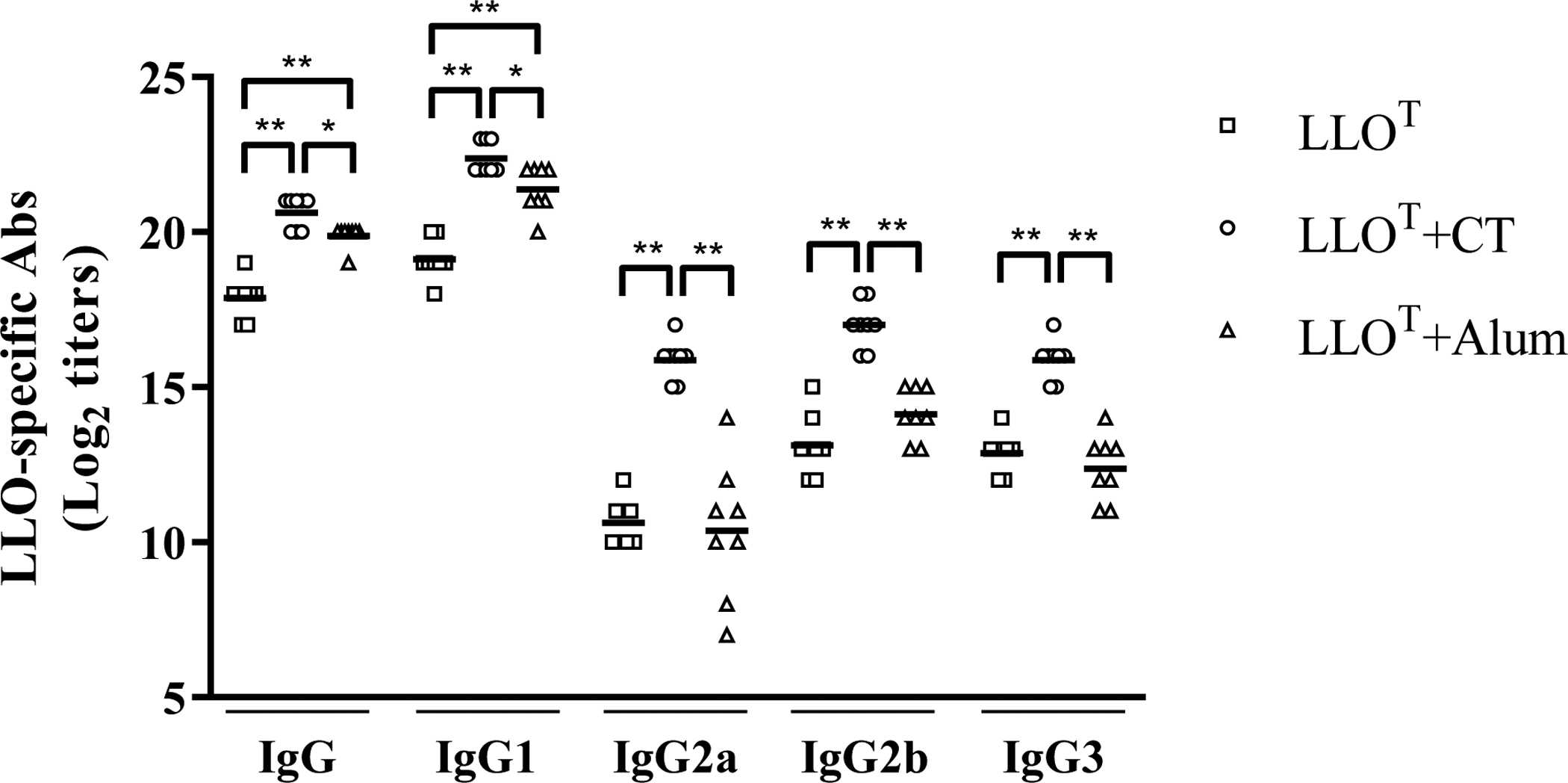
The titers of LLOT-specific IgG, IgG1 and IgG2a, IgG2b, and IgG3 were determined by ELISA in serially diluted (1:2) sera from mice immunized with LLOT, LLOT+CT (cholera toxin), or LLOT + Alum. LLO-specific IgGs in sera from mice inoculated with adjuvants alone were similar to naïve mice (data not shown). Antibody titers were determined as the last dilution of sera with an absorbance > 0.1 above that of control sera from naïve mice. Antibody titers are expressed as Log2 values for each mouse with the mean presented. Statistical significance was calculated using a one-way ANOVA, * P < 0.05, ** P < 0.01. N = titers from 8 mice for each group.
Figure 7. Immunization with LLOT plus cholera toxin generates LLO-neutralizing antibodies.
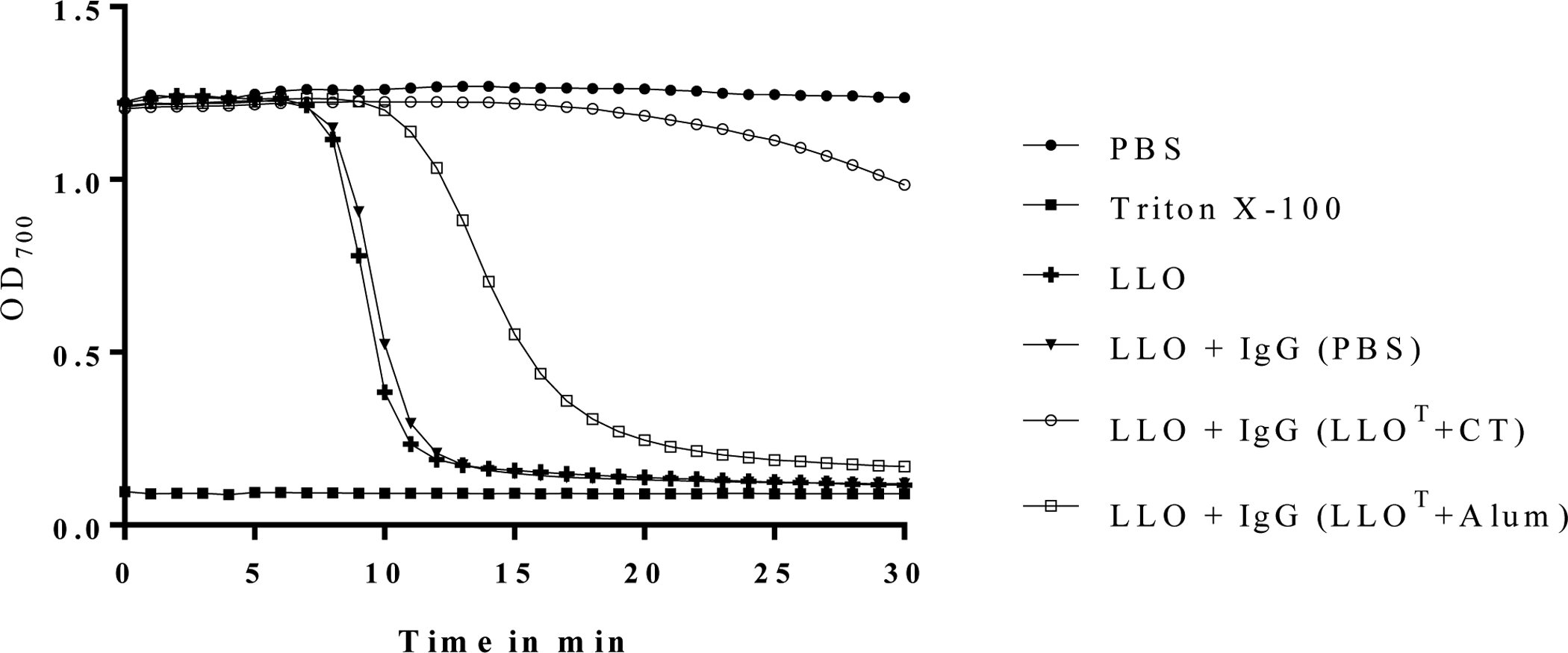
IgGs (15 μg/ml) were purified from pooled sera isolated from 8 mice per immunized group: PBS, LLOT + CT adjuvant, or LLOT + Alum adjuvant, and tested for their ability to neutralize 5 nM LLO mixed with human erythrocytes. Erythrocyte lysis of was measured at OD700 at 60 sec intervals for 30 min. As hemolysis negative and positive controls, erythrocytes were incubated with PBS or Triton X-100, respectively. Data are representative of 4 independent experiments.
Immunization with LLOT plus cholera toxin protects mice lacking mature B cells against L. monocytogenes
To establish if the production of anti-LLO neutralizing antibodies elicited by LLOT + CT had a significant role in protection against L. monocytogenes, we repeated the immunization procedure using mice that lack mature B cells (μMT−/−) in comparison to wild type mice. We observed that, regardless of treatment, μMT−/− mice were more resistant to infection compared to WT mice, as previously reported in the literature55,56. As expected, we did not detect LLO-specific IgGs in immunized μMT−/− mice (data not shown). Despite the lack of LLO-specific antibody production in μMT−/− mice, LLOT + CT still induced significant protection against L. monocytogenes (Fig. 8). These data show that the antibody response is dispensable for the vaccine-acquired protection against L. monocytogenes. However, the intrinsic increased resistance of μMT−/− mice to L. monocytogenes infection reflects that innate and/or T cell immunity differ in this model, making data interpretation complex.
Figure 8. Immunization with LLOT plus cholera toxin is protective in μMT−/− mice that lack mature B cells.
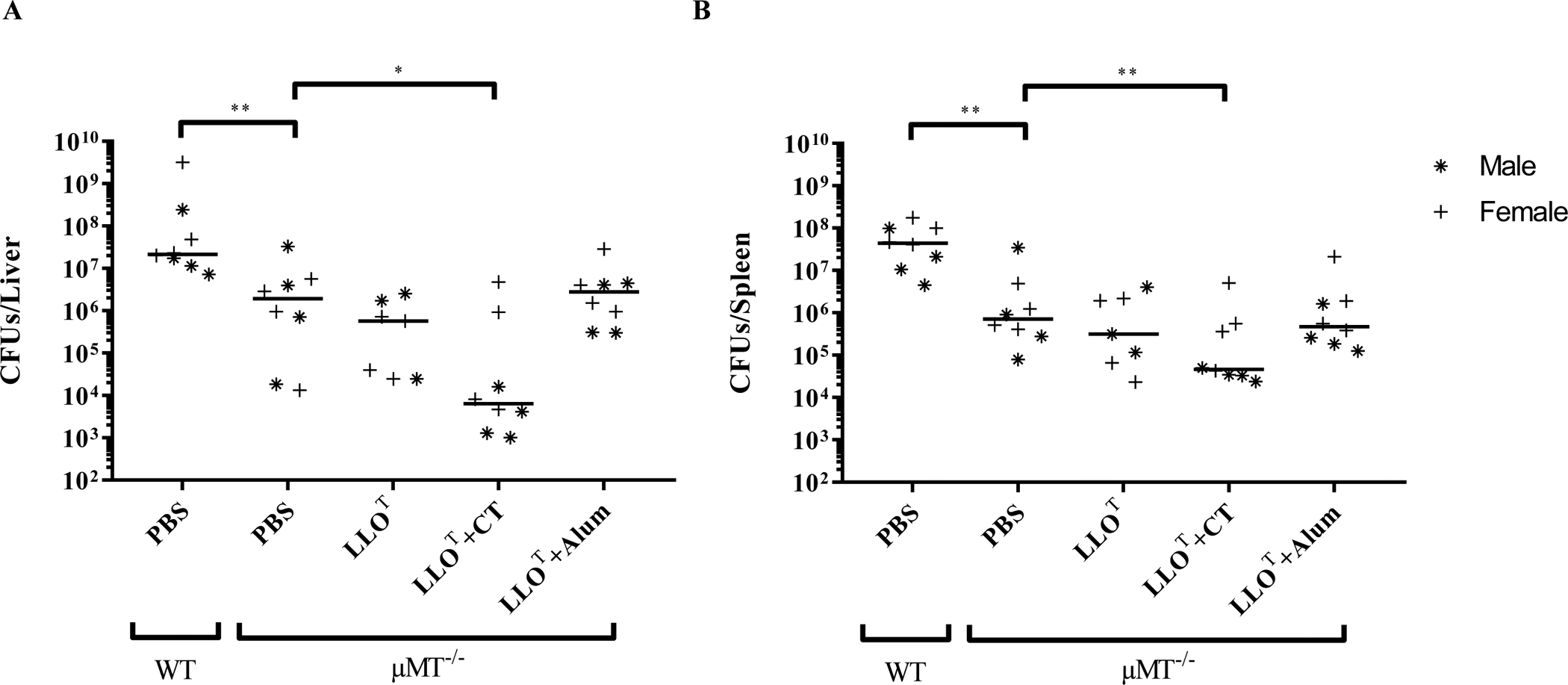
WT and μMT−/− mice (4 males and 4 females per experimental condition, with the exception of the LLOT condition that included 4 females and 3 males, were immunized at weekly intervals for 3 consecutive weeks by intraperitoneal injection of PBS (negative control), LLOT (20 μg), LLOT (20 μg) plus CT adjuvant (1 μg), or LLOT (20 μg) plus Alum adjuvant (40 μg). At day 28, mice were intravenously inoculated with 2 × 104 L. monocytogenes and sacrificed after 72 h to enumerate bacterial colony forming units (CFUs) in the liver (A) and spleen (B). Results are expressed as CFUs per organ and medians are presented. Statistical significance was calculated using a two-sided Mann-Whitney test, * P <0.05, ** P < 0.01.
Immunization with LLOT plus cholera toxin elicits a pronounced increase in Th1 responses
To compare the T cell responses elicited by the protective (LLOT + CT) and non-protective (LLOT + Alum) vaccines, splenocytes were isolated from the different immunization groups and exposed to LLOT for 5 days and stimulated with PMA and ionomycin. Splenocytes were then co-labeled with fluorescent anti-CD3 and anti-CD4 antibodies. After permeabilization, cells were labeled with fluorescent anti-cytokine antibodies. This labeling strategy allowed us to establish the profiles of the T helper responses based on cytokine production by CD3/CD4 double positive cells. Th2 cells release IL-5, IL-4, and IL-10 that support the production of antibodies. The main cytokines produced by Tfh cells (IL-21) and Th17 cells (IL-17A) also facilitate antibody production and affinity maturation. We found that immunization with LLOT plus either Alum or, CT led to similar increases in Th2, Tfh, and Th17 associated cytokines when compared to the conditions LLOT, PBS, and the adjuvants alone. Th1 responses, characterized by the production of IFN-γ, promote cell-mediated immunity, including cytotoxic CD8+ T cells and the activation of macrophages, both of which are important for protection against L. monocytogenes50. Taken together, these results indicate that the protective immunization with LLO + CT leads to the most pronounced increase in Th1-type IFN-γ responses when compared to all other conditions (Fig. 9A).
Figure 9. Analysis of T cell responses in the different immunized groups.
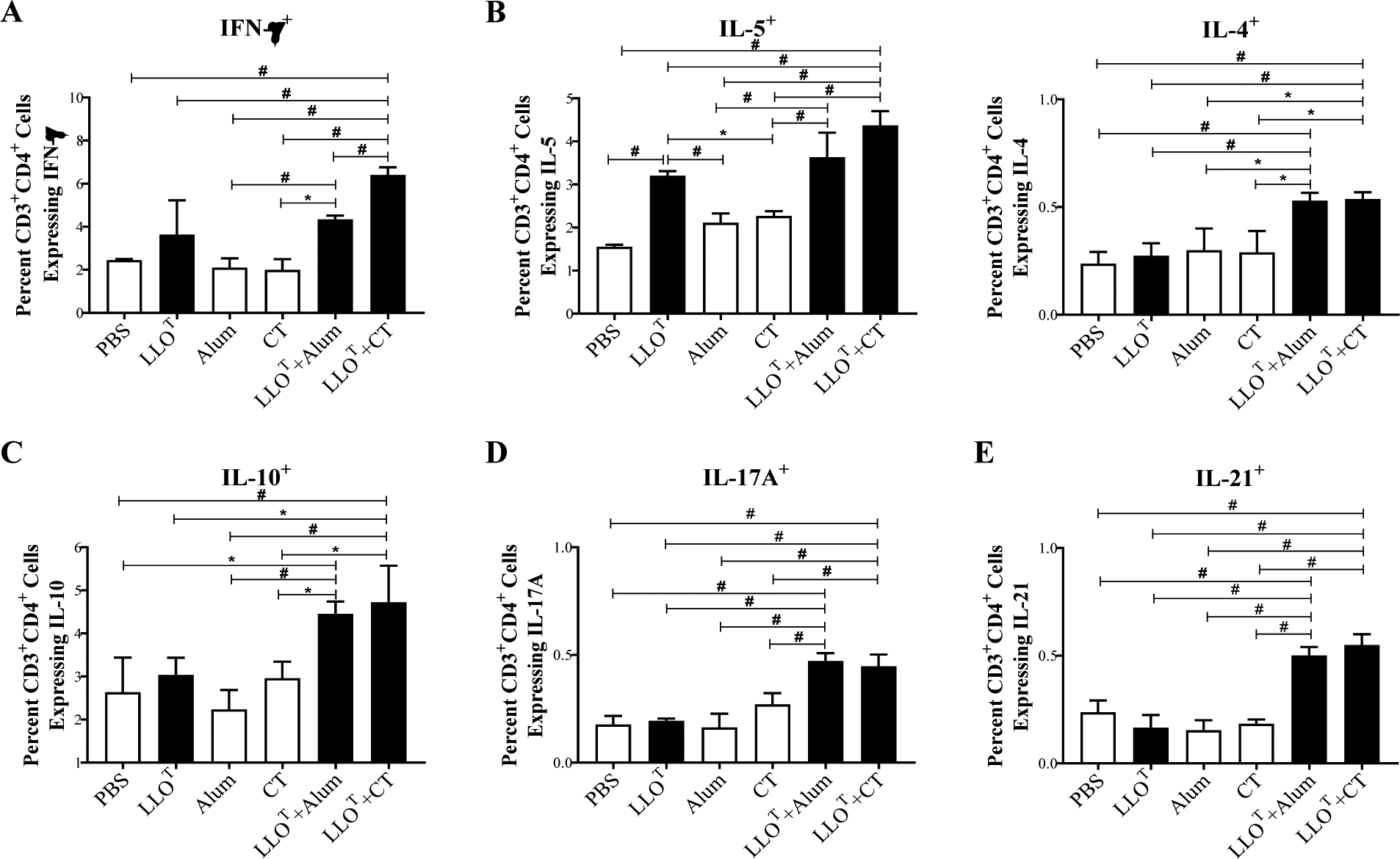
Splenocytes from the different immunized groups were isolated at day 38 after initial immunization and cultured 5 days in the presence of 5 μg/ml LLOT. Cultured splenocytes were then stimulated with PMA and ionomycin for 1 h and subsequently treated with Golgistop for 5 h. Splenocytes were then labeled with fluorescently tagged antibodies against CD3, CD4, IFN-γ, IL-5, IL-4, IL-10, IL-17a, and IL-21 and analyzed by flow cytometry. The frequencies of LLOT-specific Th1 (CD3+CD4+IFN-γ+) (A); Th2 (CD3+CD4+IL-5+, CD3+CD4+IL-4+, and CD3+CD4+IL-10+) (B and C); Th17 (CD3+CD4+IL-17A+) (D); and Tfh (CD3+CD4+IL-21+) (E) were expressed as the average percentage of positive cells for indicated cytokines ± standard deviation among the CD3/CD4 double positive cells. Statistical differences were determined by one-way ANOVA and significant differences were considered at: * p ≤ 0.05 and # p ≤ 0.005.
Effective immunization with LLOT plus cholera toxin is mediated by T cells
In order to confirm the role of T cells in the anti-L. monocytogenes protection of mice immunized with LLOT + CT, we depleted T cells after immunization by administering a cocktail of CD8- and CD4-cell-depleting antibodies, or control isotypes, 48 h before and 24 h after infection. Analysis of circulating leukocytes confirmed the efficacy of T cell depletion, whereas B cells, natural killer cells, and dendritic cells remained unaffected (supplemental Fig. 1). When isotype control antibodies were administered to mice immunized with LLOT + CT, we observed significant decreases in bacterial burden 72 h post-infection, as expected (Fig. 10). Importantly, T cell depletion post-immunization abrogated protection in the LLOT + CT group, demonstrating that T cells are required for effective immunization (Fig. 10).
Figure 10. T cells are required for the protective immunity of the LLOT+CT immunization group.
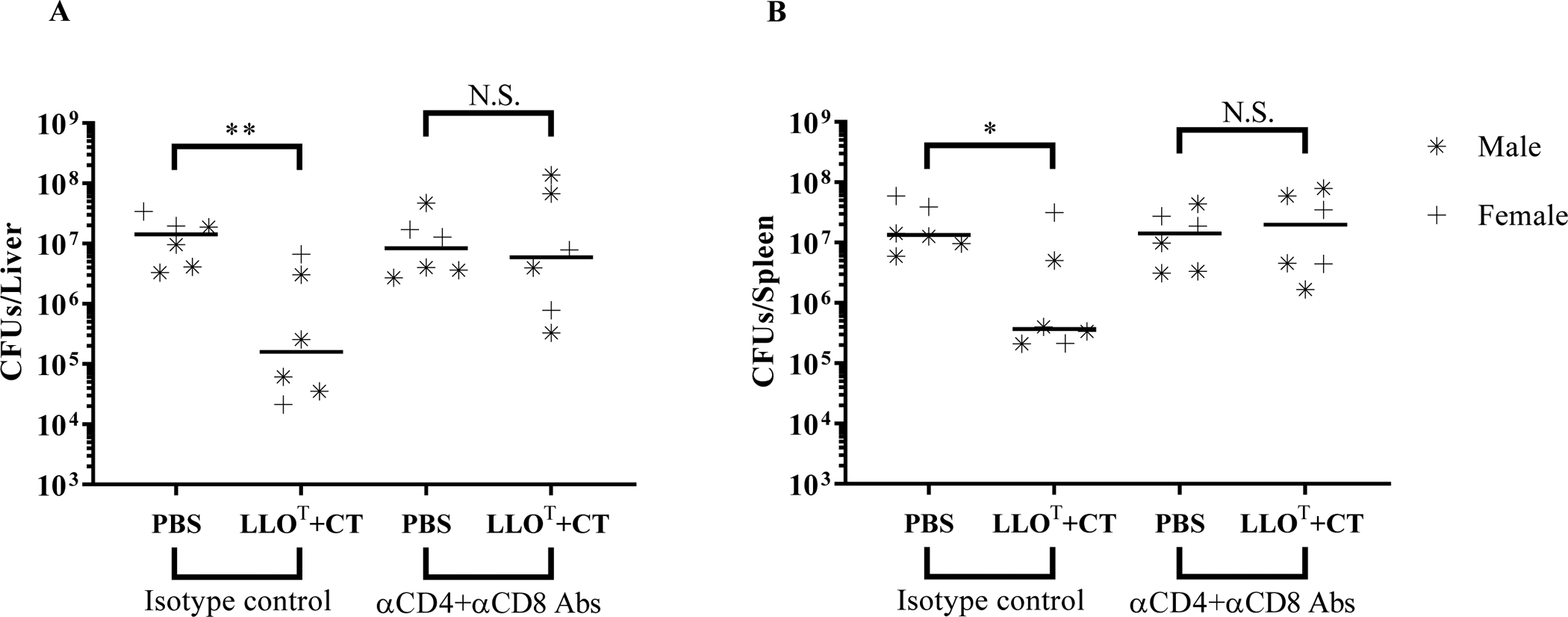
Mice were immunized at weekly intervals for 3 consecutive weeks by intraperitoneal injection of PBS (negative control), or LLOT (20 μg) plus CT (1 μg). Mice received 300 μg of CD4 plus CD8 depleting antibodies or 300 μg of corresponding isotype controls on day 26 via intraperitoneal injection (600 μg total). On day 28, mice were intravenously inoculated with 2 × 104 L. monocytogenes. Mice were given a second 100 μg dose of depleting antibodies or isotype control antibodies 24 h post-infection (200 μg total). Mice were sacrificed after 72 h of infection to collect organs and enumerate bacterial colony forming units (CFUs) in the liver (A) and spleen (B). Data are from 1 experiment with 4 males and 2 females per experimental condition. Results are expressed as CFUs per organ and medians are presented. Statistical significance was calculated using a two-sided Mann-Whitney test, N.S. = Not statistically significant, * P < 0.05 and ** P < 0.01.
Discussion
We report the generation of a full-length LLO toxoid (LLOT) in which the Thr-Leu cholesterol recognition motif in domain 4 was substituted with two glycine residues (T515G/L516G). Using LLOT and the adjuvant cholera toxin, we created a novel experimental vaccine that protects against infection by L. monocytogenes. This vaccine elicits CD4+ Th1 cells that produce IFN-γ and LLO-neutralizing antibodies. However, in this model, protective immunity involved T cells whereas, B cells were dispensable. The advantages of developing a LLOT-based subunit vaccine include safety and the fact that LLOT binds antigen-presenting cells and contains all antigenic epitopes, while LLO toxicity is abrogated.
The Threonine-Leucine motif located in domain 4 is conserved among the cholesterol-dependent cytolysin (CDC) family members and is essential for perfringolysin O (PFO), streptolysin O (SLO), pneumolysin (PLY), and intermedilysin (ILY) binding to cholesterol42,57. As expected, the present work shows that this motif is also required for LLO binding to cholesterol (Fig. 1). Most CDCs, including PFO, PLY, and SLO, bind host cells in a cholesterol-dependent fashion, i.e. they are unable to bind cholesterol-depleted cells, or to bind cells in the absence of the cholesterol recognition motif42,57. However, for a few CDCs such as ILY, host cell binding involves a protein surface receptor even though pore formation is cholesterol-dependent58,59. Importantly, LLOT is still able to bind host cells in the absence of cholesterol recognition motif and despite cholesterol depletion (Fig. 2). This suggests the existence of additional host receptor(s) for LLO, the nature of which remain(s) to be determined. As expected, LLOT displays drastically reduced hemolytic activity, which is as low as a truncated LLO variant lacking the membrane-binding domain (Fig. 3). Of note, the LLO attenuated variant LLO W492A is only 50-fold less hemolytic than native LLO, whereas LLOT is 3,000-fold less hemolytic than native LLO. The loss of toxicity, the maintenance of LLO membrane binding, and the preserved presence of T cell epitopes make LLOT an excellent candidate for anti-L. monocytogenes subunit vaccine development. In support of this strategy, vaccines against several other pathogens that produce CDCs have used detoxified variants of their respective CDCs60–64.
LLO and its derivatives display immunogenic and adjuvant properties24. When used as an adjuvant with a dengue virus antigen, a LLO variant (mutations in the consensus undecapeptide sequence, which is critical for formation of the LLO pore) increased dengue virus envelope protein-specific IgG1 and IgG2a65. This particular LLO variant was also effective as an adjuvant for tumor immunotherapy in mice26. It will be of interest to establish if LLOT, which is unable to form pores, also retains such adjuvant properties in these models. Here, we show that LLOT elicits the production of LLO-specific IgG1, IgG2a/b, IgG3 and IL-5 producing Th cells. However, LLOT alone failed to elicit protective T-cell immunity in mice challenged with L. monocytogenes and splenocytes isolated from mice immunized with LLOT alone failed to produce IFNγ when re-exposed to LLO in vitro. Therefore, in the present immunization and infection model, LLOT was not acting as an adjuvant sufficient for stimulation of protective T cell immunity.
Key players that mediate sterilizing immune responses to L. monocytogenes include CD4+ Th1 cells producing IFN-γ, which activate macrophages and CD8+ cytotoxic T cell responses29,50,66,67. Studies by Edelson et al. using the murine infection model suggested that, unlike the robust T cell responses, B cell responses and the production of antibodies were limited in response to L. monocytogenes infection51. However, the adoptive transfer of a monoclonal LLO-neutralizing antibody, but not of anti-LLO non-neutralizing antibodies, protected naïve mice against sub-lethal and lethal doses of L. monocytogenes51. This protection was not attributed to bacterial opsonization by anti-LLO antibodies, because injection of similar amounts of non-neutralizing anti-LLO antibodies that could opsonize the bacterium had no effect. Rather, the protective effect was attributed to LLO neutralization within the phagosome of cells that have internalized L. monocytogenes68. LLO neutralizing antibodies also likely abrogate the extracellular activities of LLO, such as the induction of bacterial internalization into hepatocytes (Supplemental Figure 2). These observations led to the hypothesis that, if naturally induced, LLO neutralizing antibodies would protect against L. monocytogenes. Here, cholera toxin was used as an experimental adjuvant that elicits balanced and robust T- and B- cell immune responses52. When administered in combination with LLOT, the vaccination protocol significantly protected mice against L. monocytogenes (Fig. 4), induced Th1 responses (Fig. 9), and significantly increased the production of LLO-specific IgG1, IgG2a/b, and IgG3 isotypes when compared to LLOT alone54 (Fig. 6), with IgG2a isotype class switching known to be driven by IFN-γ69. These antibodies neutralized LLO activity (Fig. 7). To tease apart the role of LLO-neutralizing antibodies from the role of Th1 cells, we also used Alum, an adjuvant known to elicit robust antibody production without concurrently inducing strong Th1 T cell responses53. However, inoculation of Alum and LLOT did not reduce infectious burden (Fig. 5) and our results were not conclusive because Alum was less efficient than cholera toxin in eliciting LLO-specific antibodies, including the IgG2a, IgG2b, and IgG3 isotypes, and LLO neutralizing Abs (Figs. 6, 7)70,71. We then used mice lacking mature B cells (μMT−/−). As previously reported, μMT−/− mice were more resistant to L. monocytogenes infection in comparison to WT55,72, which was attributed to decreased macrophage anti-listerial responses involving the stimulation of IL-10 producing B cells55. Similar observations were reported with SCID mice infected with L. monocytogenes73. Importantly, immunization of μMT−/− with LLOT + CT was still protective (Fig. 9), indicating that LLO-neutralizing antibodies are dispensable for protection against L. monocytogenes in our experimental conditions. Finally, T cell depletion experiments confirmed that T cell responses were responsible for the observed acquired protection against L. monocytogenes, and the major distinction of the LLOT + CT and LLOT + Alum immunizations was the significant increase in IFN-γ+ CD4+ T cells, indicating a Th1 dominated response. This is in agreement with the critical role of IFN-γ in the activation of CD8+ T cells and macrophages for the clearance of L. monocytogenes74.
A future developmental stage of a listeriosis vaccine using LLOT should include a potent adjuvant safer than cholera toxin. Cholera toxin has been extensively used as model adjuvant in vaccine studies in mice for many decades. Its mechanism of action (i.e., stimulation of cAMP after binding ganglioside receptors and subsequent innate responses) are well understood and thus, this adjuvant provides a solid basis for future studies. However, injection of high doses of cholera toxin can induce edema, but this was not the case in our studies where only low doses of cholera toxin were injected75–77. Although we did not observe a significant role for LLO-neutralizing antibodies in young mice infected with L. monocytogenes, future studies should test the role of Th1 T cell responses and LLO neutralizing antibodies, in combination and individually, in protective immunity in aging and pregnant murine models, corresponding to the populations that are the most susceptible to listeriosis. In particular, IgGs are transported across the placental barrier in humans78,79 and the placental yolk sac in mice80. It is a possibility that in the context of pregnancy, with the altered T cell responses in the placenta, the antibodies may have a beneficial role in protection of the mother and/or the offspring via transplacental transfer of antibodies.
Supplementary Material
Supplemental Figure 1. Monitoring T cell depletion. Mice were immunized at weekly intervals for three consecutive weeks by intraperitoneal injection of PBS (negative control), or LLOT (20 μg) plus CT (1 μg). Mice received 300 μg of CD4- plus CD8-depleting antibodies or 300 μg of corresponding isotype controls on day 26 via intraperitoneal injection (600 μg total). On day 28, mice were intravenously inoculated with 2 × 104 L. monocytogenes. Mice were given a second 100 μg dose of depleting antibodies or isotype control antibodies 24 h post-infection (200 μg total). Mice were sacrificed after 72 h of infection to enumerate bacterial CFUs (Fig.10) and whole blood was collected in heparin tubes. Red blood cells were lysed, and the remaining cells were labeled with fluorescently tagged antibodies against CD3, CD4, and CD8 to identify T cell populations (A) and CD19, CD11c, CD11b, and NK1.1 to identify other immune cell populations (B). Data are from one experiment including 4 males and 2 females per experimental condition, and are expressed as the average percentage of total live cells initially gated ± standard deviation. Statistical significance was calculated using a two-tailed Student’s t-test, ** P < 0.01, *** P<0.001.
Supplemental Figure 2: LLO neutralizing antibodies inhibit L. monocytogenes internalization into hepatocytes. HepG2 cells were incubated for 30 min with wild type (wt) or LLO-deficient L. monocytogenes (Δhly) at MOI = 5, in the presence or absence of increasing concentrations of LLO neutralizing antibodies. L. monocytogenes internalization was measured by fluorescence microscopy. Results are the normalized mean ± SEM of three independent experiments. P values were calculated using a two tails Student’s t-test (** = P <0.01).
Acknowledgments
Research reported in this manuscript was supported by the NIAID of the National Institutes of Health under award number RO1AI107250 to Stephanie M. Seveau. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no competing financial interests.
Abbreviations:
- Ab
antibody
- CDC
cholesterol-dependent cytolysin
- CFU
colony forming unit
- LLO
listeriolysin O
- CT
Cholera toxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vazquez-Boland JA, Dominguez-Bernal G, Gonzalez-Zorn B, Kreft J & Goebel W Pathogenicity islands and virulence evolution in Listeria. Microbes Infect 3, 571–584 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Teberg AJ, Yonekura ML, Salminen C & Pavlova Z Clinical manifestations of epidemic neonatal listeriosis. Pediatr Infect Dis J 6, 817–820 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Mylonakis E, Paliou M, Hohmann EL, Calderwood SB & Wing EJ Listeriosis during pregnancy: a case series and review of 222 cases. Medicine (Baltimore) 81, 260–269 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Robbins JR & Bakardjiev AI Pathogens and the placental fortress. Curr Opin Microbiol 15, 36–43, doi: 10.1016/j.mib.2011.11.006 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berche P Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog 18, 323–336, doi: 10.1006/mpat.1995.0029 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Disson O & Lecuit M Targeting of the central nervous system by Listeria monocytogenes. Virulence 3, 213–221, doi: 10.4161/viru.19586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scharff RL Economic burden from health losses due to foodborne illness in the United States. J Food Prot 75, 123–131, doi: 10.4315/0362-028X.JFP-11-058 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Pohl AM et al. Differences Among Incidence Rates of Invasive Listeriosis in the U.S. FoodNet Population by Age, Sex, Race/Ethnicity, and Pregnancy Status, 2008–2016. Foodborne Pathog Dis, doi: 10.1089/fpd.2018.2548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira LAC et al. Listeria monocytogenes in Export-approved Beef from Mato Grosso, Brazil: Prevalence, Molecular Characterization and Resistance to Antibiotics and Disinfectants. Microorganisms 8, doi: 10.3390/microorganisms8010018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamber S et al. Occurrence of Listeria monocytogenes in Ready-to-eat Meat and Poultry Product Verification Testing Samples from United States Department of Agriculture-regulated Producing Establishments, 2005–2017. J Food Prot, doi: 10.4315/JFP-20-010 (2020). [DOI] [PubMed] [Google Scholar]
- 11.McLauchlin J, Grant KA & Amar CFL Human foodborne listeriosis in England and Wales, 1981 to 2015. Epidemiol Infect 148, e54, doi: 10.1017/S0950268820000473 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard JL, Berche P & Sansonetti P Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun 52, 50–55 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seveau S Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes. Subcell Biochem 80, 161–195, doi: 10.1007/978-94-017-8881-6_9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadia S et al. The pore-forming toxin listeriolysin O mediates a novel entry pathway of L. monocytogenes into human hepatocytes. PLoS Pathog 7, e1002356, doi: 10.1371/journal.ppat.1002356 PPATHOGENS-D-11–00558 [pii] (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen BN, Peterson BN & Portnoy DA Listeriolysin O: A phagosome-specific cytolysin revisited. Cell Microbiol 21, e12988, doi: 10.1111/cmi.12988 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osborne SE & Brumell JH Listeriolysin O: from bazooka to Swiss army knife. Philos Trans R Soc Lond B Biol Sci 372, doi: 10.1098/rstb.2016.0222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamon MA, Ribet D, Stavru F & Cossart P Listeriolysin O: the Swiss army knife of Listeria. Trends Microbiol 20, 360–368, doi: 10.1016/j.tim.2012.04.006 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Geginat G, Schenk S, Skoberne M, Goebel W & Hof H A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J Immunol 166, 1877–1884, doi: 10.4049/jimmunol.166.3.1877 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Flores KG & Vivanco-Cid H Biological effects of listeriolysin O: implications for vaccination. Biomed Res Int 2015, 360741, doi: 10.1155/2015/360741 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z et al. Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+FoxP3- and CD8+ T cells. Cancer Immunol Res 2, 911–922, doi: 10.1158/2326-6066.CIR-13-0197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilert A, Baruch L, Bronshtein T & Machluf M PLGA-Listeriolysin O microspheres: Opening the gate for cytosolic delivery of cancer antigens. Biomed Microdevices 18, 23, doi: 10.1007/s10544-016-0050-6 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Miles B, Safran HP & Monk BJ Therapeutic options for treatment of human papillomavirus-associated cancers - novel immunologic vaccines: ADXS11–001. Gynecol Oncol Res Pract 4, 10, doi: 10.1186/s40661-017-0047-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radulovic S et al. Therapeutic cancer vaccines in cervical cancer: phase I study of Lovaxin-C. J BUON 14 Suppl 1, S165–168 (2009). [PubMed] [Google Scholar]
- 24.Sun R & Liu Y Listeriolysin O as a strong immunogenic molecule for the development of new anti-tumor vaccines. Hum Vaccin Immunother 9, 1058–1068, doi: 10.4161/hv.23871 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teran-Navarro H et al. Pre-clinical development of Listeria-based nanovaccines as immunotherapies for solid tumours: insights from melanoma. Oncoimmunology 8, e1541534, doi: 10.1080/2162402X.2018.1541534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallecha A et al. Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability. Clin Vaccine Immunol 20, 77–84, doi: 10.1128/CVI.00488-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowd GC et al. Listeria monocytogenes mutants defective in gallbladder replication represent safety-enhanced vaccine delivery platforms. Hum Vaccin Immunother 12, 2059–2063, doi: 10.1080/21645515.2016.1154248 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin HP, Bahey-El-Din M, Casey PG, Hill C & Gahan CG A mutant in the Listeria monocytogenes Fur-regulated virulence locus (frvA) induces cellular immunity and confers protection against listeriosis in mice. J Med Microbiol 62, 185–190, doi: 10.1099/jmm.0.049114-0 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Mackaness GB Cellular resistance to infection. J Exp Med 116, 381–406, doi: 10.1084/jem.116.3.381 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y et al. A Promising Listeria-Vectored Vaccine Induces Th1-Type Immune Responses and Confers Protection Against Tuberculosis. Front Cell Infect Microbiol 7, 407, doi: 10.3389/fcimb.2017.00407 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chavez-Arroyo A & Portnoy DA Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells? Cell Microbiol 22, e13175, doi: 10.1111/cmi.13175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng W et al. Recombinant Listeria promotes tumor rejection by CD8(+) T cell-dependent remodeling of the tumor microenvironment. Proc Natl Acad Sci U S A 115, 8179–8184, doi: 10.1073/pnas.1801910115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le DT, Dubenksy TW Jr. & Brockstedt DG Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol 39, 311–322, doi: 10.1053/j.seminoncol.2012.02.008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miles BA, Monk BJ & Safran HP Mechanistic insights into ADXS11–001 human papillomavirus-associated cancer immunotherapy. Gynecol Oncol Res Pract 4, 9, doi: 10.1186/s40661-017-0046-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flickinger JC Jr., Rodeck U & Snook AE Listeria monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines (Basel) 6, doi: 10.3390/vaccines6030048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basu P et al. A Randomized Phase 2 Study of ADXS11–001 Listeria monocytogenes-Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int J Gynecol Cancer 28, 764–772, doi: 10.1097/IGC.0000000000001235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fares E et al. Vaccine strain Listeria monocytogenes bacteremia occurring 31 months after immunization. Infection, doi: 10.1007/s15010-018-1249-7 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Calderon-Gonzalez R et al. Cellular vaccines in listeriosis: role of the Listeria antigen GAPDH. Front Cell Infect Microbiol 4, 22, doi: 10.3389/fcimb.2014.00022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen S et al. Adenovirus-based vaccine against Listeria monocytogenes: extending the concept of invariant chain linkage. J Immunol 191, 4152–4164, doi: 10.4049/jimmunol.1301290 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Del Rio E et al. A gold glyco-nanoparticle carrying a Listeriolysin O peptide and formulated with Advax delta inulin adjuvant induces robust T-cell protection against listeria infection. Vaccine 33, 1465–1473, doi: 10.1016/j.vaccine.2015.01.062 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Luo X & Cai X A combined use of autolysin p60 and listeriolysin O antigens induces high protective immune responses against Listeria monocytogenes infection. Curr Microbiol 65, 813–818, doi: 10.1007/s00284-012-0238-9 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Farrand AJ, LaChapelle S, Hotze EM, Johnson AE & Tweten RK Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci U S A 107, 4341–4346, doi: 10.1073/pnas.0911581107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michel E, Reich KA, Favier R, Berche P & Cossart P Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol Microbiol 4, 2167–2178, doi: 10.1111/j.1365-2958.1990.tb00578.x (1990). [DOI] [PubMed] [Google Scholar]
- 44.Kohda C et al. Dissociated linkage of cytokine-inducing activity and cytotoxicity to different domains of listeriolysin O from Listeria monocytogenes. Infect Immun 70, 1334–1341 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam JGT et al. Host cell perforation by listeriolysin O (LLO) activates a Ca(2+)-dependent cPKC/Rac1/Arp2/3 signaling pathway that promotes Listeria monocytogenes internalization independently of membrane resealing. Mol Biol Cell 29, 270–284, doi: 10.1091/mbc.E17-09-0561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malyala P & Singh M Endotoxin limits in formulations for preclinical research. J Pharm Sci 97, 2041–2044, doi: 10.1002/jps.21152 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Kitamura D, Roes J, Kuhn R & Rajewsky K A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350, 423–426, doi: 10.1038/350423a0 (1991). [DOI] [PubMed] [Google Scholar]
- 48.Tweten RK Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun 73, 6199–6209, doi: 10.1128/IAI.73.10.6199-6209.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenfield NJ Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1, 2876–2890, doi: 10.1038/nprot.2006.202 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhardwaj V, Kanagawa O, Swanson PE & Unanue ER Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J Immunol 160, 376–384 (1998). [PubMed] [Google Scholar]
- 51.Edelson BT, Cossart P & Unanue ER Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol 163, 4087–4090 (1999). [PubMed] [Google Scholar]
- 52.Mattsson J et al. Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsalpha in CD11b(+) DCs. Mucosal Immunol 8, 815–827, doi: 10.1038/mi.2014.111 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Oleszycka E & Lavelle EC Immunomodulatory properties of the vaccine adjuvant alum. Curr Opin Immunol 28, 1–5, doi: 10.1016/j.coi.2013.12.007 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Bonnegarde-Bernard A et al. IKKbeta in intestinal epithelial cells regulates allergen-specific IgA and allergic inflammation at distant mucosal sites. Mucosal Immunol 7, 257–267, doi: 10.1038/mi.2013.43 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horikawa M et al. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol 190, 1158–1168, doi: 10.4049/jimmunol.1201427 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly-Scumpia KM et al. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med 208, 1673–1682, doi: 10.1084/jem.20101715 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christie MP, Johnstone BA, Tweten RK, Parker MW & Morton CJ Cholesterol-dependent cytolysins: from water-soluble state to membrane pore. Biophys Rev 10, 1337–1348, doi: 10.1007/s12551-018-0448-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giddings KS, Johnson AE & Tweten RK Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci U S A 100, 11315–11320, doi: 10.1073/pnas.2033520100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawrence SL et al. Structural Basis for Receptor Recognition by the Human CD59-Responsive Cholesterol-Dependent Cytolysins. Structure 24, 1488–1498, doi: 10.1016/j.str.2016.06.017 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cowan GJ, Atkins HS, Johnson LK, Titball RW & Mitchell TJ Immunisation with anthrolysin O or a genetic toxoid protects against challenge with the toxin but not against Bacillus anthracis. Vaccine 25, 7197–7205, doi: 10.1016/j.vaccine.2007.07.040 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Paton JC et al. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun 59, 2297–2304 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jost BH, Trinh HT, Songer JG & Billington SJ Immunization with genetic toxoids of the Arcanobacterium pyogenes cholesterol-dependent cytolysin, pyolysin, protects mice against infection. Infect Immun 71, 2966–2969 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alexander JE et al. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun 62, 5683–5688 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bubeck Wardenburg J & Schneewind O Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med 205, 287–294, doi: 10.1084/jem.20072208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez-Flores KG et al. Evaluation of the safety and adjuvant effect of a detoxified listeriolysin O mutant on the humoral response to dengue virus antigens. Clin Exp Immunol 188, 109–126, doi: 10.1111/cei.12906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane FC & Unanue ER Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med 135, 1104–1112 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miki K & Mackaness GB The Passive Transfer of Acquired Resistance to Listeria Monocytogenes. J Exp Med 120, 93–103 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edelson BT & Unanue ER Intracellular antibody neutralizes Listeria growth. Immunity 14, 503–512, doi: 10.1016/s1074-7613(01)00139-x (2001). [DOI] [PubMed] [Google Scholar]
- 69.Snapper CM & Paul WE Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236, 944–947 (1987). [DOI] [PubMed] [Google Scholar]
- 70.Michaelsen TE, Kolberg J, Aase A, Herstad TK & Hoiby EA The four mouse IgG isotypes differ extensively in bactericidal and opsonophagocytic activity when reacting with the P1.16 epitope on the outer membrane PorA protein of Neisseria meningitidis. Scand J Immunol 59, 34–39 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Germann T et al. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol 25, 823–829, doi: 10.1002/eji.1830250329 (1995). [DOI] [PubMed] [Google Scholar]
- 72.Matsuzaki G, Vordermeier HM, Hashimoto A, Nomoto K & Ivanyi J The role of B cells in the establishment of T cell response in mice infected with an intracellular bacteria, Listeria monocytogenes. Cell Immunol 194, 178–185, doi: 10.1006/cimm.1999.1503 (1999). [DOI] [PubMed] [Google Scholar]
- 73.Carrero JA, Calderon B & Unanue ER Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J Exp Med 203, 933–940, doi: 10.1084/jem.20060045 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andersson A, Dai WJ, Di Santo JP & Brombacher F Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol 161, 5600–5606 (1998). [PubMed] [Google Scholar]
- 75.Pizza M et al. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19, 2534–2541, doi: 10.1016/s0264-410x(00)00553-3 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Levine MM, Kaper JB, Black RE & Clements ML New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev 47, 510–550 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shim BS et al. Development of Safe and Non-Self-Immunogenic Mucosal Adjuvant by Recombinant Fusion of Cholera Toxin A1 Subunit with Protein Transduction Domain. J Immunol Res 2018, 9830701, doi: 10.1155/2018/9830701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simister NE Placental transport of immunoglobulin G. Vaccine 21, 3365–3369, doi: 10.1016/s0264-410x(03)00334-7 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA & Carneiro-Sampaio M IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012, 985646, doi: 10.1155/2012/985646 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J et al. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol 182, 2583–2589, doi: 10.4049/jimmunol.0803247 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Monitoring T cell depletion. Mice were immunized at weekly intervals for three consecutive weeks by intraperitoneal injection of PBS (negative control), or LLOT (20 μg) plus CT (1 μg). Mice received 300 μg of CD4- plus CD8-depleting antibodies or 300 μg of corresponding isotype controls on day 26 via intraperitoneal injection (600 μg total). On day 28, mice were intravenously inoculated with 2 × 104 L. monocytogenes. Mice were given a second 100 μg dose of depleting antibodies or isotype control antibodies 24 h post-infection (200 μg total). Mice were sacrificed after 72 h of infection to enumerate bacterial CFUs (Fig.10) and whole blood was collected in heparin tubes. Red blood cells were lysed, and the remaining cells were labeled with fluorescently tagged antibodies against CD3, CD4, and CD8 to identify T cell populations (A) and CD19, CD11c, CD11b, and NK1.1 to identify other immune cell populations (B). Data are from one experiment including 4 males and 2 females per experimental condition, and are expressed as the average percentage of total live cells initially gated ± standard deviation. Statistical significance was calculated using a two-tailed Student’s t-test, ** P < 0.01, *** P<0.001.
Supplemental Figure 2: LLO neutralizing antibodies inhibit L. monocytogenes internalization into hepatocytes. HepG2 cells were incubated for 30 min with wild type (wt) or LLO-deficient L. monocytogenes (Δhly) at MOI = 5, in the presence or absence of increasing concentrations of LLO neutralizing antibodies. L. monocytogenes internalization was measured by fluorescence microscopy. Results are the normalized mean ± SEM of three independent experiments. P values were calculated using a two tails Student’s t-test (** = P <0.01).


