Fig. 1.
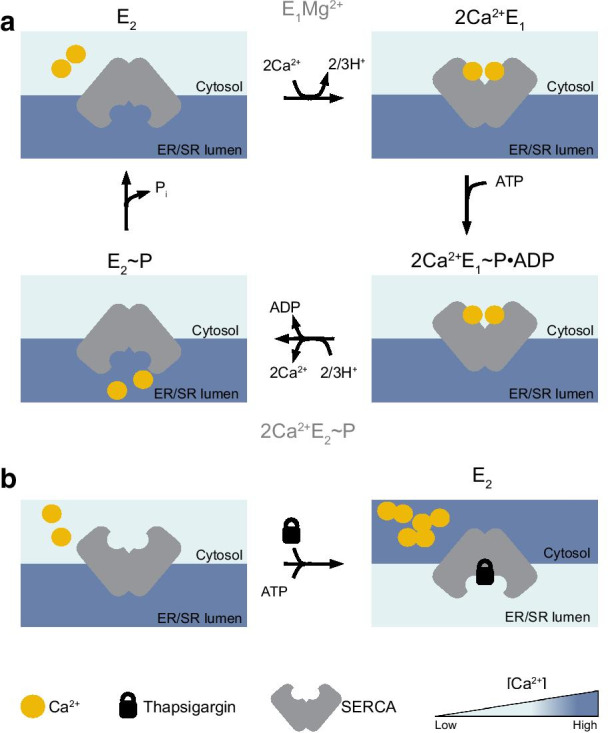
SERCA catalytic cycle. a SERCA pumps Ca2+ from the cytoplasm to the endoplasmic reticulum (ER) to create an ion gradient of ~ 10,000-fold across the cell membranes. During the catalytic cycle the Ca2+-ATPase switches between diverse oriented structures (E1 and E2) characterized by a different affinity for Ca2+ ions. The enzymatic transition between conformations catalyzes ATP in a stoichiometric ratio with Ca2+ of 1:2. E1 binds two molecules of cytoplasmic Ca2+ (2Ca2+E1, top right) and hydrolyzes one molecule of ATP to induce the high energy state of SERCA (E1 ~ P). Following the decay in ADP, E2 ~ P releases Ca2+ in the ER lumen. The cycle is closed and reopened along with the dephosphorylation of SERCA. b Thapsigargin (or other SERCA inhibitors) locks SERCA in the ground E2 state (“dead-end”) preventing Ca2+ binding and ATP hydrolysis. Thapsigargin blockade causes a continuum leakage of Ca2+ from the ER to the cytosol reverting the physiological polarization of Ca2+ across these two compartments
