Abstract
Respiratory failure is the most common cause of death in patients with corona virus disease 2019 (COVID‐19). There have been many investigations to determine predictors of bad outcomes in patients with this illness. Liver enzyme elevation has been described in hospitalized patients with severe COVID‐19; however, little is known about the significance of liver injury regarding outcomes. We conducted a retrospective chart review of 348 patients admitted with COVID‐19 in our quaternary care center. Liver injury on admission was defined based on the laboratory cutoff of aspartate aminotransferase >35 IU/L and/or alanine aminotransferase >52 IU/L. Patients were divided into two cohorts based on the presence or absence of liver injury. These cohorts were compared to assess differences in presentation, complications, and outcomes. The primary outcome was respiratory failure requiring intubation, and the secondary outcome was in‐hospital mortality. The presence of new onset liver enzyme elevation on presentation was associated with increased severity of illness, need for mechanical ventilation, and mortality. Presence of liver injury increased the chance of acute hypoxic respiratory failure requiring mechanical ventilation by 1.79 times. The degree and timeline of liver enzyme elevation during hospitalization corresponded with elevations of other inflammatory markers. Conclusion: Liver injury appears to correlate with the inflammatory syndrome caused by COVID‐19, with the degree of liver injury corresponding with severity of inflammation. We suggest early and continued monitoring of liver enzymes as they can be useful to identify patients who may need early escalation of care.
Abbreviations
- ACE‐2
angiotensin converting enzyme 2
- ACEi
angiotensin converting enzyme inhibitor
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- LDH
lactate dehydrogenase
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused global alarm. COVID‐19 was declared a pandemic by the World Health Organization on March 11, 2020. Originally from Wuhan, China, the virus has since spread to over 187 countries.( 1 ) The rapid influx of information from many countries has aimed to help clinicians better understand trends in clinical characteristics, complications, and management strategies.
COVID‐19 is primarily a respiratory illness, with severe acute hypoxic respiratory failure being the most severe complication.( 2 ) However, observational studies have also reported cardiac, renal, neurologic, and gastrointestinal involvement.( 3 , 4 ) Elevated liver enzymes have been noted to be common in patients presenting with COVID‐19, with some studies reporting an incidence up to 53%.( 5 ) More significant transaminase elevations have been seen in patients with severe illness compared to mild disease.( 6 , 7 , 8 , 9 , 10 ) A proposed hypothesis for this relates to SARS‐CoV‐2 showing an increased angiotensin converting enzyme 2 (ACE‐2) receptor affinity, similar to SARS.( 8 ) ACE‐2 receptors are found in abundance in the liver and biliary system. Notably, liver histology in patients with COVID‐19 has resemblance to liver biopsy findings in SARS.( 8 , 11 ) However, the pathophysiology of liver injury caused by the virus is not well understood. Moreover, the extent of liver injury and its impact on overall patient outcomes are yet to be elucidated.
The objectives of this study are to assess potential differences in demographics, comorbidities, severity, and outcomes in patients with COVID‐19 presenting with liver injury compared to those without liver injury.
Participants and Methods
Study Design
A retrospective chart review was conducted that included consecutive patients admitted with laboratory‐confirmed SARS‐CoV‐2 from March 10, 2020, to March 27, 2020. All enrolled patients were adults, age 18 years and above, who underwent nasopharyngeal swab testing for SARS‐CoV‐2 RNA by reverse‐transcription polymerase chain reaction (PCR) in an inpatient setting. Patients were excluded if insufficient data were available in the electronic medical record or testing was performed in the outpatient setting. This study was approved by the Institutional Review Board of Henry Ford Hospital. Written informed consent was waived by the ethics commission of the hospital for patients with emerging infectious diseases.
Data Collection
Epidemiologic, clinical, laboratory, and treatment outcome data were obtained from chart reviews. Key recorded data included signs and symptoms at presentation, medical history, laboratory findings, and treatment measures.
Definition of Variables
Liver injury was defined as aspartate aminotransferase (AST) >35 IU/L and/or alanine aminotransferase (ALT) >52 IU/L, based on laboratory cut‐off values at our institution. For comorbid conditions, “respiratory illness” included patients with chronic obstructive pulmonary disease or asthma; “kidney disease” included chronic kidney disease or end‐stage renal disease; cardiovascular disease included patients with coronary artery disease or congestive heart failure.
Statistical Analysis
Categorical variables were described by frequency and percentages, and continuous variables were described by the mean, median, and interquartile range. Patient characteristics were compared using the Student t test for continuous variables and chi‐square or Fisher’s exact tests for categorical variables. Kaplan‐Meier survival analysis and log‐rank tests were performed for patients with and without liver injury. Univariate and multivariate Cox proportional hazards model was used to identify predictors of need for mechanical ventilation during hospitalization. Receiver operating characteristic curves were created to predict mortality from the individual laboratory values using logistic regression. A repeated measures mixed model was created to determine if the time to peak AST, ALT, and D‐dimer levels differed. Pearson correlations were run to assess the relationships between these three laboratory tests. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). For all analyses, P ≤ 0.05 was considered statistically significant.
Results
A total of 366 inpatients tested positive for SARS‐CoV‐2 by nasopharyngeal PCR during the study period. Due to missing liver biochemistry tests on admission, 18 patients were excluded, leaving a study cohort of 348 patients for analysis. Patients were divided into two cohorts based on the presence (cohort 1) or absence (cohort 2) of liver injury, as defined by AST >35 IU/L and/or ALT >52 IU/L. In the overall cohort (n = 348), the average age was 61.5 ± 15.2 years, 50.3% were women, 74.4% were African Americans, and the average body mass index (BMI) was 33.6 kg/m2. Characteristics and comparisons of the two cohorts are listed in Table 1.
Table 1.
Clinical Characteristics of Patients With Liver Injury (Cohort 1) and Without Liver Injury (Cohort 2)
| Variables | Overall | Cohort 1 | Cohort 2 | P Value |
|---|---|---|---|---|
| I. Total numbert of observations (% of total) | 348 (100%) | 184 (52.9%) | 164 (47.2%) | |
| II. Demographic characteristic | ||||
| Age, years, mean ± SD | 61.5 ± 15.3 | 62.7 ± 14.7 | 60.2 ± 15.8 | 0.1 |
| Sex, number (%) | 0.001 | |||
| Female | 175 (50.3%) | 77 (41.8%) | 98 (59.8) | |
| Male | 173(49.7%) | 107(58.1%) | 66 (40.2%) | |
| Race, number (%) | 0.7 | |||
| White | 47 (13.5%) | 24 (13.0%) | 23 (14.0%) | |
| Black | 260 (74.7%) | 136 (73.9%) | 124 (75.6%) | |
| Others | 41 (11.8%) | 24 (13.0%) | 17 (10.4%) | |
| BMI, number (%) | 0.6 | |||
| <30 kg/m2 | 133 (38.2%) | 66 (35.9%) | 67 (40.9%) | |
| 30‐40 kg/m2 | 137 (39.4%) | 77 (41.8%) | 60 (36.6%) | |
| >40 kg/m2 | 78 (22.4%) | 41 (22.3%) | 37 (22.6%) | |
| III. Symptoms at admission, number (%) | ||||
| Fever | 237 (68.1%) | 129 (70.1%) | 108 (65.9%) | 0.5 |
| Cough | 261 (75.0%) | 142 (77.2%) | 119 (72.6%) | 0.4 |
| Shortness of breath | 239 (68.7%) | 129 (70.1%) | 110 (67.1%) | 0.6 |
| Chills | 149 (42.8%) | 78 (42.4%) | 71 (43.3%) | 1.0 |
| Myalgias | 145 (41.7%) | 75 (40.8%) | 70 (42.7%) | 0.8 |
| Headache | 56 (16.1%) | 30 (16.3%) | 26 (15.9%) | 1.0 |
| Nasal congestion | 74 (21.3%) | 40 (21.7%) | 34 (20.7%) | 0.9 |
| Loss of smell | 8 (2.3%) | 5 (2.7%) | 3 (1.8%) | 0.5 |
| Gastrointestinal symptoms | 183 (52.6%) | 96 (52.2%) | 87 (53.0%) | 1.0 |
| Nausea | 88 (25.3%) | 44 (23.9%) | 44 (26.8%) | 0.6 |
| Vomiting | 53 (15.2%) | 27 (14.7%) | 26 (15.9%) | 0.9 |
| Abdominal pain | 56 (16.1%) | 30 (16.3%) | 26 (15.9%) | 1.0 |
| Anorexia | 100 (28.7%) | 56 (30.4%) | 44 (26.8%) | 0.5 |
| Loss of taste | 2 (0.6%) | 2 (1.15%) | 0 (0.0%) | 0.5 |
| Diarrhea | 100 (28.7%) | 54 (29.3%) | 46 (28.0%) | 0.9 |
| IV. Comorbidities, number (%) | ||||
| Hypertension | 260 (74.7%) | 137 (74.5%) | 123 (75.0%) | 1.0 |
| Diabetes mellitus | 161 (46.3%) | 84 (45.7%) | 77 (47.0%) | 0.9 |
| Cardiovascular disease (CAD or CHF) | 84 (24.1%) | 36 (19.6%) | 48 (29.3%) | 0.05 |
| Kidney disease (CKD or ESRD) | 175 (50.6%) | 99 (54.4%) | 76 (46.3%) | 0.2 |
| Respiratory illness (COPD or asthma) | 112 (32.6%) | 56 (30.8%) | 56 (34.6%) | 0.5 |
| History of liver disease | 19 (5.5%) | 8 (4.3%) | 11 (6.7%) | 0.66 |
| Cirrhosis | 3 (0.9%) | 2 (1.1%) | 1 (0.6%) | 1.0 |
| Fatty liver on prior imaging | 37 (10.6%) | 17 (9.2%) | 20 (12.2%) | 0.08 |
| V. Medications, number (%) | ||||
| Aspirin | 123 (35.3%) | 61 (33.2%) | 62 (37.8%) | 0.4 |
| NSAIDs | 63 (18.1%) | 41 (22.3%) | 22 (13.4%) | 0.05 |
| ACEi/angiotensin receptor blockers | 80 (21.9%) | 41 (25%) | 38 (20.7%) | 0.4 |
| Statin | 147 (42.2%) | 72 (39.1%) | 75 (45.7%) | 0.3 |
| VI. Laboratory data, median (interquartile range) | ||||
| White blood cell count, K/µL | 5.8 (4.2, 7.5) | 6.1 (4.6, 7.7) | 5.5 (3.9, 7.3) | 0.05 |
| Hemoglobin, g/dL | 13.2 (11.8, 14.4) | 13.6 (12.2, 14.6) | 12.5 (11.5, 13.9) | <0.001 |
| Neutrophil count, K/µL | 4.6 (3.1, 6.7) | 4.8 (3.5, 7.2) | 4.3 (2.9, 6.2) | 0.08 |
| Lymphocyte count, K/µL | 0.9 (0.6, 1.3) | 0.9 (0.7, 1.2) | 0.9 (0.6, 1.3) | 0.6 |
| Platelet count, K/µL | 177.0 (144.0, 227.5) | 171.0 (142.0, 218.0) | 182.5 (148.0, 236.5) | 0.1 |
| AST, IU/L, mean (SD) | 49.8 (8.9) | 71.6 (59.5) | 25.3 (6.0) | <0.001 |
| ALT, IU/L, mean (SD) | 31.4 (30.5) | 43.6 (37.1) | 17.8 (9.00) | <0.001 |
| Maximum AST during admission, IU/L | 57.00 (35.00, 100.00) | 77.00 (54.00, 116.75) | 35.00 (26.00, 57.25) | <0.001 |
| Maximum ALT during admission, IU/L | 40.00 (22.00, 77.75) | 61.00 (33.00, 92.00) | 23.50 (17.75, 48.50) | <0.001 |
| Total bilirubin, mg/dL | 0.6 (0.4, 0.8) | 0.6 (0.5, 0.8) | 0.5 (0.4, 0.7) | <0.001 |
| Albumin, mg/dL | 3.5 (3.2, 3.8) | 3.5 (3.2, 3.8) | 3.5 (3.2, 3.8) | 0.6 |
| INR, mean (SD) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 0.7 |
| INR <1 | 47 (13.5%) | 23 (12.5%) | 24 (14.6%) | 0.001 |
| INR ≥1 | 226 (64.9%) | 135 (73.4%) | 91 (55.5%) | |
| No value available | 75 (21.6%) | 26 (14.1%) | 49 (29.9%) | |
| PT, seconds | 13.70 (13.10, 14.45) | 13.70 (13.10, 14.43) | 13.60 (12.90, 14.45) | 0.805 |
| PTT, seconds | 32.0 (29.0, 36.5) | 32.0 (29.0, 36.0) | 32.0 (28.0, 37.0) | 0.9 |
| Lactate, mmol/L | 1.3 (1.0, 1.8) | 1.4 (1.1, 2.0) | 1.2 (0.9, 1.6) | <0.001 |
| Procalcitonin, number (%) | ||||
| <0.25 ng/mL | 197 (56.6%) | 96 (52.2%) | 101 (61.6%) | 0.001 |
| >0.25 ng/mL | 115 (33.0%) | 76 (41.3%) | 39 (23.8%) | |
| No value available | 36 (10.3%) | 12 (6.5%) | 24 (14.6%) | |
| LDH, IU/L | 336.0 (253.0, 467.5) | 408.5 (326.0, 528.5) | 261.0 (219.0, 333.5) | <0.001 |
| Creatinine phosphokinase, IU/L | 183.0 (93.0, 441.5) | 322.0 (132.0, 644.5) | 115.5 (69.3, 218.8) | <0.001 |
| CRP, mg/dL | 9.2 (4.6, 14.3) | 11.4 (6.9, 15.7) | 6.1 (2.2, 11.2) | <0.001 |
| Ferritin, ng/mL | 537.0 (228.5, 1,181.0) | 763.0 (361.0, 1,382.8) | 344.0 (144.0, 710.5) | <0.001 |
| D‐dimer, µg/mL | 1.1 (0.7, 2.1) | 1.2 (0.8, 2.9) | 1.00 (0.5, 1.5) | <0.001 |
| High sensitivity troponin, number (%) | ||||
| <0.18 ng/L | 43 (12.4%) | 16 (8.7%) | 27 (16.5%) | 0.04 |
| >0.18 ng/L | 288 (82.8%) | 161 (87.5%) | 127 (77.4%) | |
| No value available | 17 (4.9%) | 7 (3.8%) | 10 (6.1%) | |
| VII. Inpatient complications, number (%) | ||||
| Thromboembolic event | 22 (6.3%) | 15 (8.2%) | 7 (4.3%) | 0.2 |
| Septic shock | 66 (19.0%) | 44 (23.9%) | 22 (13.4%) | 0.02 |
| AKI requiring dialysis | 24 (7.0%) | 16 (8.8%) | 8 (5.0%) | 0.2 |
| Intensive care unit stay | 136 (39.1%) | 96 (52.2%) | 40 (24.4%) | <0.001 |
| Ventilator‐dependent respiratory failure | 120 (34.5%) | 85 (46.2%) | 35 (21.3%) | <0.001 |
| Length of stay, mean days | 11 | 14 | 9 | <0.001 |
| VIII. Inpatient medications, number (%) | ||||
| Antibiotic use | 277 (79.4%) | 156 (84.7%) | 121 (73.8%) | 0.03 |
| Systemic steroids | 225 (64.8%) | 135 (73.4%) | 90 (55.2%) | 0.001 |
| Hydroxychloroquine | 279 (80.2%) | 156 (84.8%) | 123 (75.0%) | 0.03 |
| Remdesivir or tocilizumab | 32 (9.2%) | 23 (12.5%) | 9 (5.5%) | 0.04 |
Abbreviations: AKI, acute kidney injury; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ESRD, end‐stage renal disease; INR, international normalized ratio; NSAID, nonsteroidal anti‐inflammatory drug; PT, prothrombin time; PTT, partial thromboplastin time.
Clinical Presentation
The most common presenting symptoms were cough in 78% of patients (n = 261), fever in 68.1% (n = 237), and shortness of breath in 68.7% (n = 239). Gastrointestinal (GI) symptoms were present in 52.6% (n = 183) of patients; GI symptoms included diarrhea (28.7%), anorexia (28.7%), nausea (25.3%), vomiting (15.2%), and abdominal pain (16.1%). Additional reported signs and symptoms included chills (42.8%), myalgias (41.7%), nasal congestion (21.3%), headache (16.1%), loss of smell (2.3%), and loss of taste (0.6%). Clinical symptoms on presentation were not significantly different between cohort 1 and cohort 2 (Table 1).
New Onset Liver Injury
Patients with new onset liver injury (cohort 1, n = 184 [52.8%]) were compared to patients without liver injury (cohort 2, n = 164 [47.1%]). There were no patients with baseline elevations of liver enzymes. Patients with liver injury had a mean age of 62.7 ± 14.7 years, with approximately 50% being over the age of 65. Patients in cohort 1 were more commonly men (58.2% vs. 40.2%; P = 0.001), and there was no difference in race or BMI between the two cohorts.
Regarding comorbidities, patients presenting with liver injury were more likely to have a history of cardiovascular disease compared to those without liver injury (29.3% vs. 19.6%; P = 0.047), but there was no significant difference in the presence of hypertension (74.5% vs. 75%), diabetes (45.7% vs. 47%), and kidney disease (54.4% vs. 46.3%). In the overall cohort, information collected on home medications showed that 37.5% of patients were on an ACE inhibitor (ACEi) or angiotensin receptor blocker, 33.2% were on aspirin, 22.3% were on nonsteroidal anti‐inflammatory drugs, and 9.1% were on statins. Following a review of imaging present before the current hospitalization, 23.5% of patients had abdominal imaging, with 20.7% of those patients noted to have some degree of baseline hepatic steatosis.
Patients with liver injury had significantly higher levels of total bilirubin, inflammatory markers (lactate dehydrogenase [LDH], C‐reactive protein [CRP], creatinine phosphokinase [CPK], ferritin, D‐dimer), troponin, and lactate at presentation compared to those without liver injury (Table 1). AST levels on admission ranged from 9 to 601 IU/L, while ALT levels ranged from 4 to 380 IU/L. Peak AST and ALT levels during hospitalization positively correlated with the peak D‐dimer (AST and D‐dimer: r = +0.19, P = 0.0007; ALT and D‐dimer: r = +0.29, P < 0.001). Number of days to peak AST, ALT, and D‐dimer from the day of admission were 5.8, 5.3, and 6 days, respectively (Fig. 1).
FIG. 1.
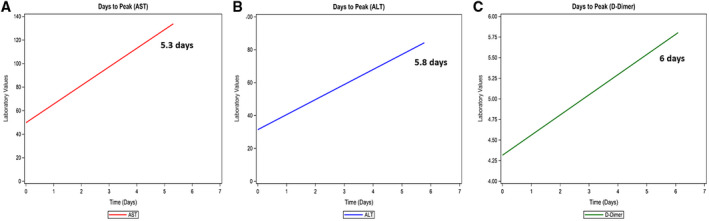
Relationship between laboratory values and peak levels. (A) Mean number of days to peak AST. (B) Mean number of days to peak ALT. (C) Mean number of days to peak D‐dimer levels. The average time to peak levels do not significantly differ (P = 0.26).
Treatment and Complications
Patients were treated with one or more therapy during hospitalization. Out of 348 patients, 80.2% received hydroxychloroquine (n = 279), 79.3% empiric antibiotics (n = 276), and 64.8% systemic steroids (n = 225). Regarding experimental therapies, 8% (n = 28) and 2% (n = 8) of patients received tocilizumab and remdesivir, respectively.
In cohort 1, patients were more frequently treated with hydroxychloroquine (84.8% vs. 75%; P = 0.03), systemic steroids (73.4% vs. 55.2%; P = 0.01), empiric antibiotics (84.2% vs. 73.8%; P = 0.027), tocilizumab (11.4% vs. 4.3%; P = 0.025), and remdesivir (2.7% vs. 1.8%; P = 0.84) compared to cohort 2.
Patients presenting with new‐onset liver injury were more likely to develop acute kidney injury requiring initiation of renal replacement therapy (8.8% vs. 5%; P = 0.24), septic shock (23.9% vs. 13.4%; P = 0.018), ventilator‐dependent respiratory failure (46.2% vs. 21.3%; P = 0.001), or need for intensive care unit admission (52.2% vs. 24.4%; P = 0.001). The length of hospital stay was significantly longer for patients with liver injury (14 days vs. 9 days; P < 0.001).
Ventilator‐Dependent Respiratory Failure
On univariate analysis, liver injury was a predictor for ventilator‐dependent respiratory failure, along with other variables (Table 2). When adjusted for age, sex, BMI, kidney disease, ferritin, CRP, D‐dimer, alkaline phosphatase, bilirubin, lactate, and albumin, liver injury remained a significant predictor for ventilator‐dependent respiratory failure (Fig. 2; Table 2).
Table 2.
Univariate and Multivariate Analysis for Predictors of Ventilator‐Dependent Respiratory Failure
| Variables | HR | 95% CI | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Presence of liver injury | 2.41 | 1.63, 3.58 | 0.001 |
| Age | 1.63 | 1.13, 2.34 | 0.009 |
| Female | 0.45 | 0.31, 0.66 | 0.001 |
| BMI (30‐40) kg/m2 | 1.73 | 1.10, 2.72 | 0.017 |
| Kidney disease | 1.86 | 1.28, 2.70 | 0.001 |
| Total bilirubin >1.2 mg/dL | 1.84 | 1.03, 3.28 | 0.038 |
| ALP >140 IU/L | 3.09 | 1.56, 6.11 | 0.001 |
| Albumin >3.2 g/dL | 0.47 | 0.32, 0.69 | 0.001 |
| Leukocytosis | 1.06 | 1.01, 1.12 | 0.017 |
| Elevated LDH | 1.00 | 1.00, 1.00 | 0.001 |
| CRP >10 mg/dL | 2.47 | 1.68, 3.63 | 0.001 |
| Ferritin >500 ng/dL | 2.53 | 1.69, 3.77 | 0.001 |
| D‐dimer >1 µg/dL | 1.71 | 1.15, 2.54 | 0.008 |
| Procalcitonin >0.25 ng/mL | 2.27 | 1.57, 3.25 | 0.001 |
| Lactate >1 mmol/L | 2.02 | 1.15, 3.54 | 0.014 |
| Multivariate analysis | |||
| Presence of liver injury | 1.79 | 1.17, 2.73 | 0.008 |
| Male | 1.94 | 1.31, 2.88 | 0.001 |
| BMI >40 kg/m2 | 2.17 | 1.30, 3.63 | 0.003 |
| Albumin >3.2 gm/dL | 2.12 | 1.42, 3.15 | <0.001 |
Abbreviations: ALP, alkaline phosphatase; CI, confidence interval; HR, hazard ratio.
FIG. 2.
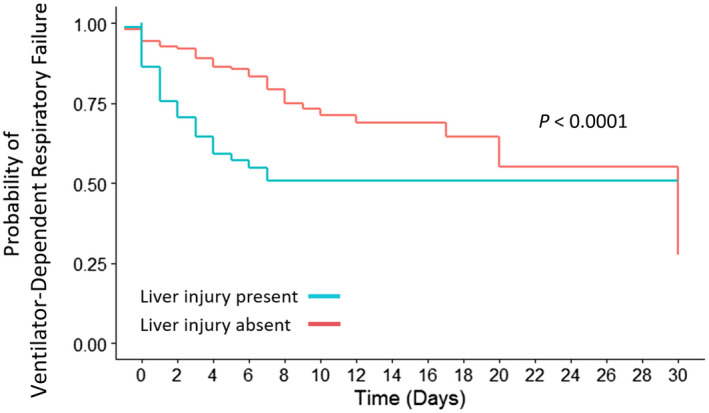
Kaplan‐Meier curve for probability of ventilator‐dependent respiratory failure in the presence or absence of liver injury on admission.
In‐Hospital Mortality
At the time of manuscript preparation, 77 out of 348 patients (22.1%) had died. Overall, 26% (n = 48) of patients died in cohort 1 and 17% (n = 29) of patients died in cohort 2; 20 patients were still hospitalized, the majority (n = 18) of which were in cohort 1. Outcomes (death or discharge) of these patients (n = 20) were not available at the time of analysis. For patients with definitive outcomes (death or discharged alive), a significantly higher number of patients with liver injury died compared to those without liver injury (28.9% vs. 17.9%; P = 0.018) (Fig. 3). Using logistic regression, increased admission AST levels; peak AST, peak ALT, and total bilirubin levels; and decreased albumin levels were individually associated with increased risk of mortality (Table 3; Fig. 4).
FIG. 3.
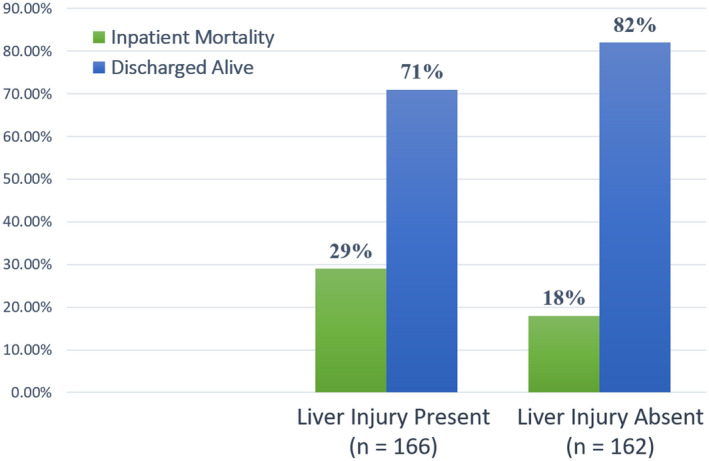
Rates of mortality in patients with definitive outcomes according to the presence or absence of liver injury. Twenty patients were excluded due to continued hospitalization.
Table 3.
Liver Function Abnormalities as Predictors of Mortality
| Laboratory Value | AUC | Odds Ratio | 95% CI | P Value |
|---|---|---|---|---|
| AST (1 unit increase) IU/L | 0.59 | 1.011 | 1.003, 1.018 | 0.006 |
| ALT (1 unit increase) IU/L | 0.50 | 1.002 | 0.989, 1.015 | 0.8 |
| Peak AST (1 unit increase) IU/L | 0.76 | 1.007 | 1.003, 1.01 | <0.001 |
| Peak ALT (1 unit increase) IU/L | 0.63 | 1.005 | 1.002, 1.008 | 0.003 |
| Total bilirubin (1 unit increase) mg/dL | 0.57 | 1.997 | 1.033, 3.862 | 0.04 |
| Albumin (1 unit increase) g/dL | 0.62 | 0.5 | 0.276, 0.845 | 0.01 |
Abbreviations: AUC, area under the curve; CI, confidence interval.
FIG. 4.
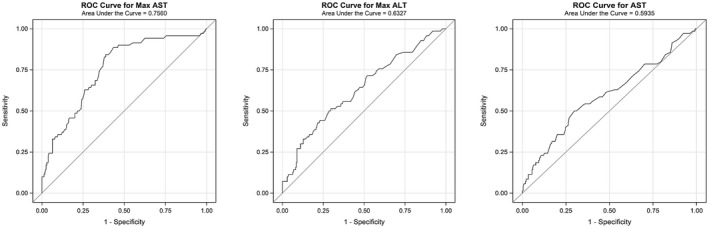
Area under the ROC curve for peak AST, peak ALT, and initial AST levels for predicting mortality. Abbreviation: ROC, receiver operating characteristic.
Discussion
In hospitalized patients with COVID‐19, the presence of new‐onset liver enzyme elevation on initial blood testing was associated with an increased severity of illness, need for mechanical ventilation, and heightened mortality rates.
In China and Italy, despite an initial decline in incidence of COVID‐19, mortality rates continued to rise.( 12 ) Our study population experienced 22% inpatient mortality (n = 77), with higher death rates noted in patients with liver injury (28.9% vs. 17.9%; P = 0.018). Our analysis showed that initial AST elevation was associated with a higher chance of death, with an AST level of 44 IU/L having a specificity of 70% and sensitivity of 50%. For every 1 unit increase in AST, there was an observed increased risk of death by approximately 1%; the same was not true for ALT (Table 3). A recent study has shown that AST elevation is followed by ALT elevation, which may explain our observation.( 13 ) Higher elevations may correspond to more end‐organ damage, which may lead to poorer outcomes. When looking at peak liver injury and increased risk of death, there was a significant association; however, there remained a considerable number of patients (n = 20) who were still hospitalized with outcomes yet to be determined. Given that increased severity is associated with longer hospitalization,( 14 ) a short‐term follow‐up may not be ideal to assess survival in these patients.
Importantly, transaminase elevation as a surrogate for liver injury was found to be an independent predictor of acute hypoxic respiratory failure requiring mechanical ventilation. Studies have shown that respiratory failure is the most common cause of death in these patients.( 2 ) Therefore, our findings can be useful in identifying those at higher risk for mechanical ventilation.
With published data and experience caring for COVID‐19, the medical community has learned that cytokine‐induced inflammatory changes and secondary infections can complicate the course of this illness. Procalcitonin on admission, which is elevated in bacterial infections, was found to be elevated in 41.3% of patients with liver injury (P = 0.001). These patients were also more likely to develop septic shock (23.9% vs. 13.4%; P = 0.018). This suggests that patients with mild transaminase elevations are at high risk of secondary bacterial infection and therefore should be identified for early initiation of antibiotics.
Hypercoagulability has been found to be prevalent in patients with COVID‐19, with many reporting significant rates of venous thromboembolic events and clotting of dialysis catheters.( 15 ) Mild elevations in liver enzymes on presentation were associated with higher international normalized ratio during hospitalization (73.4% vs. 55.5%; P = 0.001), hinting that certain patients have a predisposition to liver failure. However, there is an absence of association between liver injury and abnormal prothrombin time and partial thromboplastin time. This suggests that hypercoagulability is more likely attributed to disseminated intravascular coagulation due to severity of illness rather than liver dysfunction.
We considered whether the presence of liver injury might vary depending on comorbidities and predisposing factors. Looking at the available data, there does not appear to be an increased risk of liver injury during COVID‐19 in patients with a history of chronic liver disease.( 14 ) We only had 3 patients with cirrhosis in our study population, and therefore we could not appropriately analyze this effect. The presence of risk factors for metabolic syndrome (hypertension, diabetes, high BMI, or prior fatty liver on imaging) did not appear to be more common in patients who developed liver injury. Similarly, age did not show any predisposition.
Much attention has focused on home ACEi use because the virus acts through ACE‐2 receptors on multiple organs, including the liver.( 16 ) In our study, there was no correlation between the use of ACEi and liver injury (25% vs. 20.7%; P = 0.47). Taking these findings into consideration, liver injury appears to be independent of age, comorbid conditions, ACEi use, or baseline liver function. We suspect that liver injury in COVID‐19 is likely related to virus‐induced inflammation rather than exacerbation of an underlying process.
The degree of transaminase elevation correlated with the level of inflammation in our patients with COVID‐19. Our study confirmed a demonstrated association of liver injury with elevated CRP.( 14 ) In addition, we showed a positive correlation between liver injury and elevations in D‐dimer, ferritin, LDH, and CPK. In essence, liver function testing can be used as a surrogate for monitoring of inflammation.
There are currently no proven treatments for COVID‐19, and many options are investigational or available for compassionate use in those who are critically ill. In this study, patients with liver injury had an increased likelihood of receiving antibiotics, steroids, and experimental therapy. Moreover, patients who had liver injury on admission were noted to have higher liver enzyme elevations during hospitalization. As some treatment options may exclude patients with liver dysfunction, knowing which patients are likely to develop worse transaminases is important as it allows physicians to consider treatment options early if needed.
The main limitation of this study is the retrospective study design as the data collection is at‐risk of human errors and inaccurate clinical documentation. Some degree of misclassification of history and symptoms may be present, although all laboratory tests, including the SARS‐CoV‐2 PCR, were done at our hospital.
In conclusion, liver injury seems to be related to the inflammatory syndrome caused by COVID‐19. The presence of early liver injury is associated with increased length of hospitalization, severity of disease, need for mechanical ventilation, and mortality.
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co‐first authorship.
- 1. Johns Hopkins‐University Center for Systems Science and Engineering . COVID‐19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Updated daily. Accessed March 2020.
- 2. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asadi‐Pooya AA, Simani L. Central nervous system manifestations of COVID‐19: a systematic review. J Neurol Sci 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, et al. Clinical characteristics of 82 cases of death from COVID‐19. PLoS One 2020;15:e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang Y, Yang R, Xu Y, Gong P. Clinical characteristics of 36 non‐survivors with COVID‐19 in Wuhan, China. medRxiv 2020; 10.1101/2020.02.27.20029009. [DOI] [Google Scholar]
- 8. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. JAMA 2020;323:1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID‐19: abnormal liver function tests. J Hepatol 2020; 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Our World in Data . Coronavirus pandemic (COVID‐19). https://ourworldindata.org/coronavirus. Updated daily. Accessed March 2020.
- 13. Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology 2020; 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan Z, Chen L, Li J, Cheng X, Jingmao Y, Tian C, et al. Clinical features of COVID‐19‐related liver functional abnormality. Clin Gastroenterol Hepatol 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID‐19. Am J Hematol 2020;95:834‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, et al. Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus‐infected pneumonia. [in Chinese] Zhonghua Gan Zang Bing Za Zhi 2020;28:100‐106. [DOI] [PubMed] [Google Scholar]


