Chronic viral hepatitis is associated with substantial morbidity and mortality worldwide. Our study shows good comparability of point shear wave elastography (pSWE) and transient elastography. pSWE is a promising tool for the prediction of liver‐related events in patients with viral hepatitis, particularly in patients with cHCV.

Abstract
Chronic viral hepatitis is associated with substantial morbidity and mortality worldwide. The aim of our study was to assess the ability of point shear‐wave elastography (pSWE) using acoustic radiation force impulse imaging for the prediction of the following liver‐related events (LREs): new diagnosis of HCC, liver transplantation, or liver‐related death (hepatic decompensation was not included as an LRE). pSWE was performed at study inclusion and compared with liver histology, transient elastography (TE), and serologic biomarkers (aspartate aminotransferase to platelet ratio index, Fibrosis‐4, FibroTest). The performance of pSWE and TE to predict LREs was assessed by calculating the area under the receiver operating characteristic curve and a Cox proportional‐hazards regression model. A total of 254 patients with a median follow‐up of 78 months were included in the study. LRE occurred in 28 patients (11%) during follow‐up. In both patients with hepatitis B virus and hepatitis C virus (HCV), pSWE showed significant correlations with noninvasive tests and TE, and median pSWE and TE values were significantly different between patients with LREs and patients without LREs (both P < 0.0001). In patients with HCV, the area under the receiver operating characteristic curve for pSWE and TE to predict LREs were comparable: 0.859 (95% confidence interval [CI], 0.747‐0.969) and 0.852 (95% CI, 0.737‐0.967) (P = 0.93). In Cox regression analysis, pSWE independently predicted LREs in all patients with HCV (hazard ratio, 17.9; 95% CI, 5.21‐61‐17; P < 0.0001) and those who later received direct‐acting antiviral therapy (hazard ratio, 17.11; 95% CI, 3.88‐75.55; P = 0.0002). Conclusion: Our study shows good comparability between pSWE and TE. pSWE is a promising tool for the prediction of LREs in patients with viral hepatitis, particularly those with chronic HCV. Further studies are needed to confirm our data and assess their prognostic value in other liver diseases.
Abbreviations
- ACLF
acute‐on‐chronic liver failure
- ALT
alanine aminotransferase
- APRI
aspartate aminotransferase to platelet ratio index
- ARFI
acoustic radiation force impulse
- AST
aspartate aminotransferase
- AUROC
area under the receiver operating characteristic curve
- cHBV
chronic hepatitis B virus
- cHCV
chronic hepatitis C virus
- CI
confidence interval
- DAA
direct‐acting antiviral
- FIB‐4
Fibrosis‐4
- GT
genotype
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- LRE
liver‐related event
- PC
platelet count
- pSWE
point shear‐wave elastography
- TE
transient elastography
Chronic viral hepatitis is a serious global health burden, accounting for approximately 1.46 million deaths worldwide.( 1 ) More than 90% of these deaths are due to the sequelae of infections with hepatitis B virus (HBV) and hepatitis C virus (HCV), including cirrhosis, liver failure, and hepatocellular carcinoma (HCC).( 2 )
In patients with chronic HBV (cHBV) infection, persistent viral replication is associated with progressive liver disease and HCC.( 3 ) However, long‐term nucleos(t)ide treatment, which aims at maximal suppression of viral replication, is associated with a decreased risk of liver‐related events (LREs).( 4 ) For patients with chronic HCV (cHCV), the development of direct‐acting antivirals (DAAs) has been a major breakthrough over the past 5 years, and viral cure can now be achieved most patients.( 5 )
Despite the success of antiviral treatments, a substantial proportion of patients already have advanced liver disease at the time of diagnosis and are at risk of developing liver‐related complications despite permanent clearance of the virus from the bloodstream. Therefore, early identification of patients at risk is of major clinical importance, and management and follow‐up of these patients has become a core component of our daily clinical practice.
In patients with chronic viral hepatitis, noninvasive tools such as serum markers and/or ultrasound‐based elastography methods have become the standard of care for the diagnosis of liver fibrosis and cirrhosis, whereas liver biopsy is only recommended in selected cases.( 6 , 7 , 8 , 9 ) Transient elastography (TE) was the first ultrasound‐based method to be used for noninvasive liver fibrosis assessment.( 10 ) Since then, TE has been evaluated extensively in patients with chronic viral hepatitis( 10 , 11 , 12 ) and proven to be useful in predicting hepatic complications, such as HCC, portal hypertension–related complications, and mortality.( 13 , 14 , 15 , 16 , 17 ) Furthermore, TE has been proposed as an innovative tool to help categorize liver tumors.( 18 ) More recently, similar results were obtained from patients with nonviral chronic liver disease.( 19 , 20 )
Liver stiffness measurement by means of acoustic radiation force impulse (ARFI) imaging was a more recent addition to the elastography market.( 21 ) Several studies have shown its clinical applicability and comparability to histology and other noninvasive methods, including TE, for the assessment of liver fibrosis.( 22 )
The aim of the present study was to assess the usefulness of point shear‐wave elastography (pSWE) using ARFI for the prediction of LREs in patients with cHBV or cHCV infection. TE and noninvasive serum markers, including APRI, Fibrosis‐4 (FIB‐4) and FibroTest, were available for method comparisons.
Patients and Methods
Selection of Patients
For the present study, all consecutive patients with cHBV or cHCV infection enrolled in three prospective clinical trials between 2008 and 2012 in the University Hospital Frankfurt outpatient clinic were included.( 21 , 23 , 24 ) Patients from other participating centers, enrolled in one of the three trials, were not included in the present study (Supporting Fig. S1). Chronicity of viral hepatitis was defined as the presence of hepatitis B surface antigen and HBV DNA or presence of HCV antibodies and HCV RNA in serum for greater than 6 months at the time of study inclusion. None of the patients received antiviral therapy at the time of study inclusion. Patients with cHBV infection were treatment‐naive to nucleos(t)ide or interferon treatment, whereas patients with cHCV infection were either treatment‐naive or treatment‐experienced.
Patients with past or active malignancies, patients younger than 18 years of age, pregnant women, and patients with HBV or HCV or human immunodeficiency virus coinfection were excluded. Patients were also excluded if they consumed more than 40 g of alcohol per day in males and 20 g per day in females, respectively. Patients with signs of decompensated liver disease (ascites, hepatic encephalopathy, variceal bleeding, hepatorenal syndrome) and/or significant portal hypertension (medium or large esophageal varices) were also excluded.
Written, informed consent was obtained from all patients, and the ethics committee of the University Hospital Frankfurt approved the studies.
Noninvasive Liver Fibrosis Measurement
All patients had undergone liver biopsy within the past 15 months before study inclusion. Liver fibrosis stages were semi‐quantitatively evaluated using the Metavir scoring system.( 25 ) Liver fibrosis was staged from F0 to F4, depending on the severity of the manifestation. In most patients with cirrhosis, the diagnosis was established based on typical ultrasound and/or magnetic resonance imaging findings.
Liver stiffness measurement was performed using pSWE (Siemens Healthineers, Erlangen, Germany) using ARFI and TE (FibroScan; Echosens, Paris, France). At least 10 pSWE and TE measurements were obtained, and median values were calculated according to the manufacturers’ instructions and as described.( 21 , 23 , 24 ) pSWE was integrated into a Siemens ACUSON S2000 machine, and results were reported as meters per second. TE results are reported in kilopascals.
Both measurements were performed in the right lobe of the liver, through the intercostal space, after a minimum of 6 hours of fasting on the same day. Measurement failure was defined as the acquisition of fewer than 10 valid measurements and considered reliable if a success rate of 60% or higher and an interquartile range of 30% or lower were reached. In this study, we included only patients from the per‐protocol analysis (i.e., only patients with reliable measurements); therefore, no additional sensitivity analysis was performed.( 21 , 23 , 24 )
In addition, serum fibrosis markers, including aspartate aminotransferase (AST) to platelet ratio index (APRI), FIB‐4 index, and FibroTest (BioPredictive, Paris, France) were performed on the same day as liver stiffness measurements. The APRI score was calculated as follows: (ASTULN × 100)/PC, where ASTULN is the upper limit of the normal AST value and PC is the platelet count, in 109 cells per liter.( 26 ) The upper limits of normal AST concentrations in female and male patients were 35 IU/mL and 50 IU/mL, respectively. The FIB‐4 score was calculated as follows: (age × AST)/(PC × √ALT).( 27 ) FibroTest results were computed on the BioPredictive website (biopredictive.com) using the following serum and clinical parameters: haptoglobin, alfa‐2 macroglobulin, apoprotein A1, bilirubin, gamma‐glutamyltransferase, alanine aminotransferase, age, and sex. Child‐Turcotte‐Pugh and Model for End‐Stage Liver Disease scores were assessed by clinical examination, laboratory parameters, and results of abdominal ultrasound evaluation at the time of study inclusion.
Follow‐up
Patients were included in the study from the day of written, informed consent, and followed until death, liver transplantation, or last contact. Patients who underwent liver transplantation were excluded from all further analyses from the day of transplantation. All follow‐up data were retrospectively and anonymously analyzed from electronic hospital charts.
Study Endpoint
The primary purpose of this study was to evaluate the use of pSWE for the prediction of the first of the following three LREs that occurred during follow‐up: new diagnosis of HCC, liver transplantation, or liver‐related death. Note, hepatic decompensation was not considered as an LRE in this analysis.
Statistical Analyses
Continuous variables are shown as mean ± SD, and categorical variables are shown as frequencies and percentages. Spearman’s rank correlations were used to perform nonparametric correlation analyses. The performance of pSWE and TE for predicting LREs was assessed by calculating and comparing the time‐dependent area under the receiver operating characteristic curve (AUROC) with 95% confidence intervals (CIs).( 28 ) As in this study, patients with different follow‐up times were analyzed, and a time‐dependent model was chosen.
For patients with HCV, predictors of LREs were determined using a univariate Cox proportional‐hazards regression model. LREs were counted as an event, and all baseline parameters were included in this model. For assessment of independent predictors of survival, a multivariate Cox proportional‐hazards regression model with forward stepwise (likelihood ratio) entry was applied. Because the number of LREs was small among patients with HBV, no regression model was applied.
All statistical analyses were performed using Statistical Product and Service Solutions Statistics (SPSS Statistics) (version 22.0; IBM, New York, NY); R (version 3.6.1; R Core Team, R Foundation for Statistical Computing, Vienna, Austria; package used: timeROC version 0.3); and BiAS (version 10.03; Epsilon‐Verlag, Darmstadt, Germany). P < 0.05 was considered statistically significant.
Results
A total of 254 patients (183 with cHCV infection and 71 with cHBV infection) were included in the study. All patients had viremic HCV or HBV infection and were untreated at the time of study inclusion. Baseline characteristics of the overall study cohort of patients with cHCV and cHBV infection are summarized in Table 1. In brief, most patients (58%) were male, and the median age was 47 years. Patients with HCV and HBV significantly differed with regard to age and baseline laboratory values: Patients with HCV had more active hepatitis and more advanced liver fibrosis in histology, TE, pSWE, and other laboratory value–based fibrosis scores. Median follow‐up of the entire cohort was 78 months (range, 1‐125 months).
TABLE 1.
Characteristics and Baseline Parameters of All Included Patients
| Characteristics | All Patients (n = 254) | Patients With cHBV (n = 71) | Patients With cHCV (n = 183) | P Value |
|---|---|---|---|---|
| Age in years, median (range) | 47 (18‐80) | 36 (18‐80) | 50 (19‐77) | <0.001 |
| Male sex, n (%) | 147 (57.9) | 39 (54.9) | 108 (59.0) | 0.574 |
| Laboratory values | ||||
| ALT, U/L, mean ± SD | 84 ± 124 | 51 ± 42 | 97 ± 142 | <0.001 |
| AST, U/L, mean ± SD | 63 ± 64 | 37 ± 17 | 74 ± 72 | <0.001 |
| Bilirubin, mg/dL, mean ± SD | 0.8 ± 0.8 | 0.6 ± 0.3 | 0.9 ± 0.9 | 0.02 |
| INR, mean ± SD | 1.08 ± 0.14 | 1.06 ± 0.08 | 1.08 ± 0.16 | 0.537 |
| PC, mean ± SD | 192 ± 68 | 212 ± 52 | 185 ± 72 | 0.007 |
| HBV DNA, mean log10 IU/mL ± SD | NA | 7.2 ± 7.7 | NA | NA |
| HCV DNA, mean log10 IU/mL ± SD | NA | NA | 6.5 ± 6.7 | NA |
| HCV GT, n (%) | NA | NA | 181 | NA |
| 1a and other GT1 subtypes | NA | NA | 112 (61.9) | NA |
| 1b | NA | NA | 31 (16.9) | NA |
| 2 | NA | NA | 9 (4.9) | NA |
| 3 | NA | NA | 22 (12.0) | NA |
| 4, 5, 6, and mixed genotypes | NA | NA | 7 (3.8) | NA |
| Metavir fibrosis stage | ||||
| F0‐F2, n (%) | 182 (71.7) | 63 (88.7) | 119 (65.0) | <0.001 |
| F3/F4, n (%) | 72 (28.3) | 8 (11.3) | 64 (35.0) | <0.001 |
| Elastography | ||||
| pSWE, m/s, median (range) | 1.23 (0.82‐3.9) | 1.12 (0.84‐2.96) | 1.3 (0.82‐3.9) | <0.001 |
| TE, kPa, median (range) | 6.6 (2.0‐75.0) | 5.1 (3.2‐30) | 7.1 (2.0‐75.0) | <0.001 |
| Serum fibrosis markers | ||||
| APRI, mean ± SD | 1.12 ± 1.86 | 0.42 ± 0.22 | 1.38 ± 2.13 | <0.001 |
| FIB‐4, mean ± SD | 2.56 ± 3.55 | 1.01 ± 0.48 | 3.16 ± 4.01 | <0.001 |
| FibroTest absolute, mean ± SD | 0.46 ± 0.29 | 0.28 ± 0.19 | 0.54 ± 0.3 | <0.001 |
| Antiviral therapy initiated, n (%) | 182 (71.7) | 27 (38.0) | 155 (84.7) | <0.001 |
| Outcome | ||||
| Any LRE, n (%) | 28 (11.0) | 4 (5.6) | 24 (13.1) | 0.079 |
| HCC, n (%) | 16 (6.3) | 2 (2.8) | 14 (7.7) | 0.248 |
| Liver transplantation, n (%) | 8 (3.1) | 0 (0) | 8 (4.4) | 0.110 |
| Liver‐related death, n (%) | 18 (7.1) | 3 (4.2) | 15 (8.2) | 0.414 |
| Follow‐up time, months, median (range) | 78 (1‐125) | 83 (1‐108) | 76 (1‐125) | 0.252 |
Abbreviations: ALT, alanine aminotransferase; INR, international normalized ratio; NA, not applicable.
Among patients with HCV, 78% (n = 143) of patients were infected with HCV genotype (GT) 1, and 5% (n = 9), 13% (n = 24), and 4% (n = 7) were infected with HCV GT 2, 3, and 4, respectively. The mean baseline HCV RNA was 6.0 log10 IU/mL. A total of 26% (n = 47) of patients had received unsuccessful interferon‐based therapy before study inclusion. Antiviral therapy was initiated according to the guidelines at the time in 84.7% (n = 155) of patients during follow‐up. LREs occurred in 24 patients and included 14 HCCs and 15 deaths following acute‐on‐chronic liver failure (ACLF) as well as eight liver transplantations.
Among patients with HBV, the mean baseline HBV DNA was 4.3 ± 1.7 IU/mL. Antiviral therapy was initiated in 42% (n = 30) of patients during follow‐up. Antiviral agents included lamivudine, entecavir, or tenofovir. LREs occurred in four patients and included two HCCs and two deaths following ACLF.
Patients with LREs in the overall cohort as well as patients with HCV only were significantly older and had more advanced liver fibrosis assessed by TE, pSWE, and conventional liver fibrosis tests (APRI, FIB‐4, and FibroTest; Table 2).
TABLE 2.
Liver Stiffness and Fibrosis Assessment in Patients With and Without LREs
| Characteristics | All Patients With LRE (n = 28) | All Patients Without LRE (n = 226) | P Value | Patients With cHCV and LRE (n = 24) | Patients With cHCV Without LRE (n = 159) | P Value |
|---|---|---|---|---|---|---|
| Age in years, median (range) | 56 (26‐80) | 44 (18‐70) | <0.001 | 55 (38‐77) | 49 (19‐70) | <0.001 |
| Male sex, n (%) | 19 (67.9) | 128 (56.6) | 0.418 | 17 (70.8) | 91 (57.2) | 0.267 |
| Metavir fibrosis stage | ||||||
| F0‐F2, n (%) | 3 (11.7) | 179 (79.2) | <0.001 | 2 (8.3) | 117 (73.6) | <0.001 |
| F3/F4, n (%) | 25 (89.3) | 47 (20.8) | <0.001 | 22 (91.7) | 42 (26.4) | <0.001 |
| Elastography | ||||||
| pSWE, median (range) | 2.38 (0.88‐3.9) | 1.19 (0.82‐3.42) | <0.001 | 2.57 (0.88‐3.9) | 1.25 (0.82‐ 3.42) | <0.001 |
| TE, median (range) | 21.9 (2.0‐75.0) | 6.23 (2.2‐50.5) | <0.001 | 26.2 (2.0‐75) | 6.7 (2.2‐50.5) | <0.001 |
| Serum fibrosis markers | ||||||
| APRI, mean ± SD | 3.87 ± 3.79 | 0.77 ± 1.05 | <0.001 | 4.43 ± 3.81 | 0.92 ± 1.22 | <0.001 |
| FIB‐4, mean ± SD | 9.05 ± 6.54 | 1.76 ± 1.77 | <0.001 | 10.26 ± 6.27 | 2.09 ± 2.0 | <0.001 |
| FibroTest absolute, mean ± SD | 0.84 ± 0.17 | 0.42 ± 0.28 | <0.001 | 0.91 ± 0.05 | 0.49 ± 0.28 | <0.001 |
Performance of pSWE in the Total Cohort
Overall, 72 patients had F3/F4 fibrosis (Supporting Table S1). The median velocity of pSWE was 1.23 m/s (range, 0.82‐3.9). pSWE showed significant correlation with histology (0.69), TE (0.76), APRI (0.58), FIB‐4 (0.62), and FibroTest (0.58) (P < 0.0001 each). Of the patients, 28 experienced LREs, and 3 had Metavir F0‐F2 fibrosis in liver biopsy. Each of those 3 patients developed HCC as LREs. Median pSWE and TE values were significantly different between patients with LREs and patients without LREs (both P < 0.0001; Table 2). Figure 1 illustrates the time to LREs in the overall cohort. The mean time to LREs was 40 months (range, 3‐108 months).
FIG. 1.
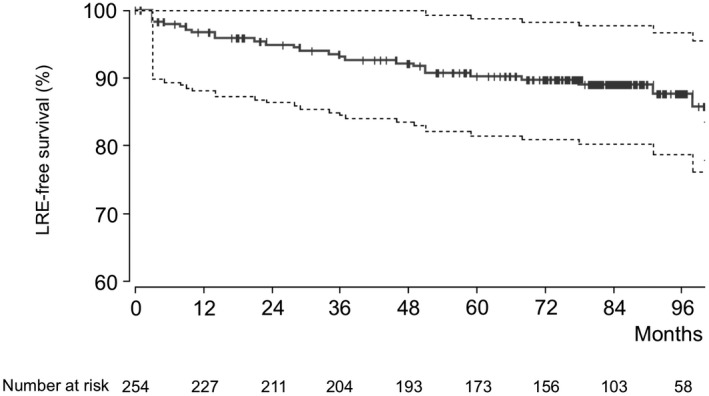
Kaplan‐Meier curve illustrating the time to LREs in the overall cohort with respective 95% CIs. The mean time to an LRE was 40 months (range, 3‐108 months).
The AUROC of pSWE and TE for the prediction of LREs was 0.839 (95% CI, 0.716‐0.944) and 0.855 (95%, CI 0.737‐0.943), respectively. The optimal cutoff with the highest sum of sensitivity and specificity (Youden cutoff) for pSWE and TE was 1.68 m/s and 10.5 kPa, respectively (Fig. 2A). The difference in AUROC between the two methods was not statistically significant (P = 0.87).
FIG. 2.
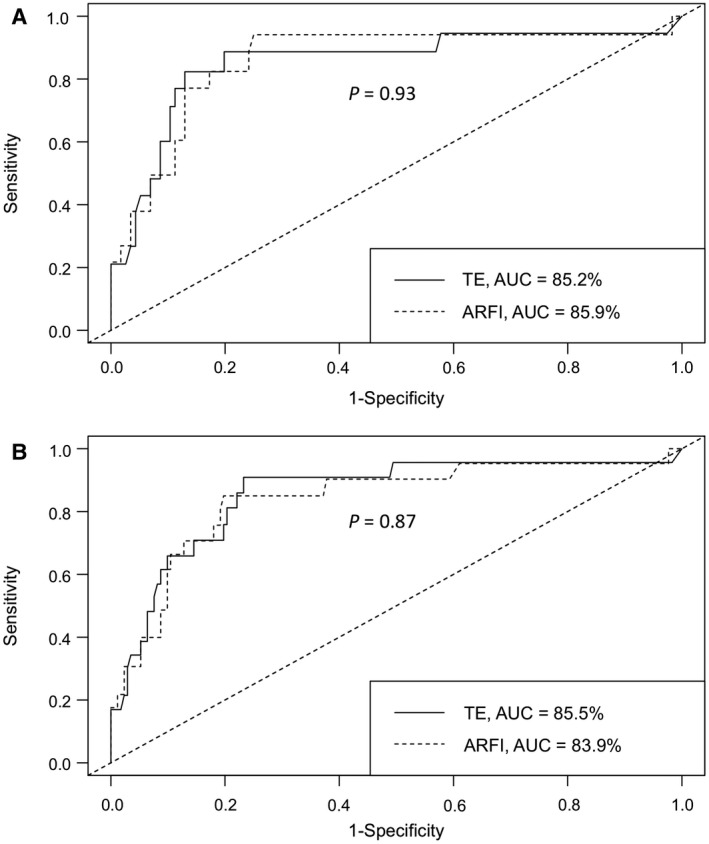
Comparison of receiver operating characteristic curves (FibroScan vs. ARFI) to predict liver LREs within 5 years in patients with cHCV (P = 0.93, for difference of receiver operating characteristic curves) (A) and in all patients (both HBV and HCV groups; P = 0.87) (B). Abbreviations: AUC, area under the curve; ROC, receiver operating characteristic.
Performance of pSWE in Patients With HCV Infection
The median pSWE velocity was 1.3 m/s (range, 0.82‐3.90). pSWE showed significant correlation with histology (0.76), TE (0.81), APRI (0.65), FIB‐4 (0.68), and FibroTest (0.63; P < 0.0001 each). Median pSWE and TE values were significantly different between patients with LREs and patients without LREs (both P < 0.0001; Table 2).
The AUROC of pSWE and TE for the prediction of LREs was 0.859 (95% CI, 0.747‐0.969) and 0.852 (95% CI, 0.737‐0.967), respectively. The optimal cutoff with the highest sum of sensitivity and specificity (Youden cutoff) for pSWE and TE was 1.68 m/s and 17.3 kPa, respectively. The difference in AUROC between the two methods was not statistically significant (P = 0.93; Fig. 2B).
Next, we performed Cox regression analyses. We calculated separate regression models for pSWE and TE, respectively. Here, pSWE (hazard ratio [HR], 17.9; 95% CI, 5.21‐61‐17; P < 0.0001) and TE (HR, 14.52; 95% CI, 5.65‐37.30; P < 0.0001) were independently associated with LREs (Table 3). As expected, HCV therapy was associated with a decreased risk of LREs (HR, 0.14 and 0.17; 95% CI, 0.05‐0.40 and 0.06‐0.49; P < 0.001 in multivariate analysis). We therefore created a second model assessing the same parameters in patients with HCV who received antiviral therapy with DAAs. Again, pSWE (HR, 17.11; 95% CI, 3.88‐75.55; P = 0.0002) and TE (HR, 11.02; 95% CI, 3.32‐31.78; P < 0.0001) remained independent variables predicting LREs (Table 4).
TABLE 3.
Univariate and Multivariate Cox Regression Analysis for LREs in Patients With cHCV Infections
| Variables (All Patients With HCV) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.02 (0.97‐1.08) | 0.371 | — | — |
| Female sex | 0.36 (0.13‐0.96) | 0.042 | 0.37 (0.14‐0.98) | 0.044 |
| HCV therapy | 0.14 (0.05‐0.40) | 0.0002 | 0.12 (0.05‐0.34) | <0.0001 |
| pSWE | 15.72 (4.48‐55.10) | <0.0001 | 17.9 (5.21‐61.17) | <0.0001 |
| Age | 1.02 (0.97‐1.08) | 0.460 | — | — |
| Female sex | 0.39 (0.15‐1.01) | 0.052 | 0.38 (0.15‐0.99) | 0.048 |
| HCV therapy | 0.17 (0.06‐0.49) | 0.0009 | 0.15 (0.06‐0.39) | 0.0001 |
| TE | 12.81 (4.79‐34.28) | <0.0001 | 14.52 (5.65‐37.30) | <0.0001 |
Baseline parameters were included in univariate analysis; only statistically significant factors in univariate analysis are depicted in this table and were included in multivariate analysis. Age was forced into the model.
TABLE 4.
Univariate and Multivariate Cox Regression Analysis for LREs in Patients With cHCV Who Received DAA Therapy
| Variables (Patients With HCV With Therapy) | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age | 1.03 (0.97‐1.09) | 0.359 | — | — |
| Female sex | 0.53 (0.17‐1.66) | 0.278 | — | — |
| pSWE | 14.22 (3.17‐63.79) | 0.0005 | 17.11 (3.88‐75.55) | 0.0002 |
| Age | 1.03 (0.97‐1.09) | 0.293 | — | — |
| Female sex | 0.51 (0.16‐1.60) | 0.251 | — | — |
| TE | 9.33 (3.18‐27.34) | <0.0001 | 11.02 (3.82‐31.78) | <0.0001 |
Baseline parameters were included in univariate analysis; only statistically significant factors in univariate analysis are depicted in this table and were included in the multivariate analysis. Age was forced into the model.
pSWE in Patients With HBV Infection
A total of 8 patients with HBV infections had F3/F4 fibrosis. The median velocity of pSWE was 1.12 m/s (range, 0.84‐2.96). The median liver stiffness according to TE was 5.1 kPa (range, 3.2‐30.0). pSWE showed significant correlations with TE, FIB‐4 and histology, with r values of 0.57, 0.35 and 0.36, respectively (all P < 0.05).
Median pSWE and TE values were not significantly different between patients with LREs and patients without LREs (both P = 0.068). However, the number of patients with HBV was low. No AUROC analysis and no regression analyses were performed in patients with HBV infection, as there were only four LREs in this group.
Discussion
In our study, we retrospectively analyzed data from three prospective clinical trials conducted between 2008 and 2012 in the University Hospital Frankfurt outpatient clinic. We were able to show that pSWE was comparable to TE to predict important LREs, such as HCC development, liver transplantation or liver‐related death, in patients with chronic viral hepatitis. Moreover, pSWE was an independent predictor of LREs in patients with cHCV.
Over the past decade, several ultrasound‐based elastography methods and serum markers have been evaluated for the assessment of liver fibrosis and are now implemented into daily clinical practice. In patients with chronic viral hepatitis, noninvasive fibrosis assessment is now widely considered the standard of care, whereas liver biopsy may only be performed in patients with unexplained discordance of imaging and/or laboratory results or suspected additional comorbidities.( 6 , 9 , 29 , 30 )
TE has been studied extensively for the staging of liver fibrosis and has shown excellent diagnostic accuracy for the diagnosis of cirrhosis, independent of the underlying liver disease.( 10 , 30 , 31 ) TE has also been evaluated as a tool to predict LREs in patients with chronic viral hepatitis, including esophageal varices, HCC, and liver‐related death.( 32 , 33 ) However, with TE, only motion mode (M‐mode) images can be obtained to localize the optimal measurement site. Moreover, studies have shown that liver inflammation and/or extrahepatic cholestasis may be associated with an increase in liver stiffness, which may disguise the true degree of fibrosis.( 34 , 35 ) Moreover, TE cannot be applied in patients with ascites.
pSWE has the potential to increase the availability of elastography methods in a wider, less specialized setting, as it is integrated into a brightness mode (B‐mode) ultrasound machine and can be performed during standard ultrasound examination of the liver.( 21 ) So far, most studies of pSWE have focused on the staging of liver fibrosis. For this indication, pSWE has shown comparable results with TE and other noninvasive tests, with excellent reproducibility.( 21 , 24 , 36 ) However, the use of pSWE for the prediction of long‐term complications of chronic viral hepatitis has rarely been studied to date.
In our study, we assessed the predictive value of pSWE in patients with cHCV and cHBV who had been included in three prospective clinical trials between 2008 and 2012.( 21 , 23 , 24 )
We found that pSWE correlated well with histology, TE, and serum fibrosis markers. The AUROCs of pSWE and TE for the prediction of LREs were comparable for patients with HCV and when both groups (HBV‐infected and HCV‐infected patients) were combined.
As expected, both patients with cHBV and cHCV who had higher liver stiffness at baseline were more likely to suffer from LREs. pSWE independently predicted LREs in all patients with HCV and in the group of patients with HCV who later received DAA therapy. This supports the use of pSWE during regular ultrasound examination at first presentation in patients with viral hepatitis, especially those with HCV infections. The use of elastography methods that are integrated into B‐mode ultrasound probes may promote a more widespread use in clinical routine, particularly outside specialized centers, thus supporting the World Health Organization–led campaign to combat hepatitis B and C and to reach their elimination by 2030.( 2 )
Given the relatively small number of patients with cHBV included in our study, it is not surprising that we were unable to assess the value of pSWE to predict LREs. Moreover, disease activity was relatively mild in patients with cHBV, which most likely also explains the small number of LREs. Yet, when both the HCV and HBV groups were combined, robust results were observed. We also observed rather high CIs in several analyses due to the modest sample size of our study. Not enough data were available to unambiguously identify patients with ACLF during follow‐up as an additional LRE. Further studies to implement ACLF or hepatic decompensations as an LRE and to improve the precision of the association are needed.
Taken together, our study shows good comparability of pSWE with established methods, including TE and serum fibrosis markers. Therefore, pSWE may not only be used for the staging of liver fibrosis but also for the prediction of disease progression, which may in turn trigger intensified screening and surveillance efforts.
Supporting information
Fig S1
Table S1
Acknowledgment
We thank Lisa Hernandez Sampere, Johannes Vermehren, Mireen Friedrich‐Rust, and Marcus M. Mücke for planning the study; Lisa Hernandez Sampere, Natalie Filmann, and Marcus M. Mücke for performing the analyses; Lisa Hernandez Sampere, Marcus M. Mücke, and Johannes Vermehren for writing the manuscript; and all authors for collecting the data, assessing and interpreting the data, and reading, revising, and approving the final version of the manuscript.
Potential conflict of interest: Dr. Vermehren is on the speaker’s bureau for Gilead and AbbVie. Dr. Waidmann consults for, is on the speakers’ bureau for, and received grants from BMS and Ipsen. He consults and is on the speakers’ bureau for Bayer, Celgene, Novartis, Roche, and Shire. He consults for and received restricted funds for investigator‐initiated trials from Merck. He consults for and is investigator for Incyte and MSD. He received grants from and received restricted funds for investigator‐initiated trials from Medac. He consults for Amgen, Eisai, and Falk. He received grants from AbbVie, and Merck Serano. He is an investigator for Basilea. Dr. Zeuzem consults for, advises, and is on the speakers’ bureau for AbbVie and Gilead. He consults for Janssen. He is on the speakers’ bureau for Merck/MSD.
References
- 1. Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Combating hepatitis B and C to reach elimination by 2030. https://www.who.int/hepatitis/publications/hep‐elimination‐by‐2030‐brief/en/. Published May 2016. Accessed September 25, 2019. [Google Scholar]
- 3. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65‐73. [DOI] [PubMed] [Google Scholar]
- 4. Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol 2015;62:956‐967. [DOI] [PubMed] [Google Scholar]
- 5. Vermehren J, Park JS, Jacobson IM, Zeuzem S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J Hepatol 2018;69:1178‐1187. [DOI] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461‐511. [DOI] [PubMed] [Google Scholar]
- 7. American Association for the Study of Liver Diseases‐Infectious Diseases Society of America (AASLD‐IDSA) HCV Guidance Panel . Hepatitis C guidance 2018 update: AASLD‐IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis 2018;67:1477‐1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis (Hoboken) 2018;12:33‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver . EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 10. Friedrich‐Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta‐analysis. Gastroenterology 2008;134:960‐974. [DOI] [PubMed] [Google Scholar]
- 11. Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, FibroTest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343‐350. [DOI] [PubMed] [Google Scholar]
- 12. Chon YE, Choi EH, Song KJ, Park JY, Kim DY, Han K‐H, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta‐analysis. PLoS One 2012;7:e44930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fung J, Lai CL, Seto WK, Wong DK, Yuen MF. Prognostic significance of liver stiffness for hepatocellular carcinoma and mortality in HBeAg‐negative chronic hepatitis B. J Viral Hepat 2011;18:738‐744. [DOI] [PubMed] [Google Scholar]
- 14. Jung KS, Kim SU, Ahn SH, Park YN, Kim DY, Park JY, et al. Risk assessment of hepatitis B virus‐related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2011;53:885‐894. [DOI] [PubMed] [Google Scholar]
- 15. Robic MA, Procopet B, Metivier S, Péron JM, Selves J, Vinel JP, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol 2011;55:1017‐1024. [DOI] [PubMed] [Google Scholar]
- 16. Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009;49:1954‐1961. [DOI] [PubMed] [Google Scholar]
- 17. Erman A, Sathya A, Nam A, Bielecki JM, Feld JJ, Thien H‐H, et al. Estimating chronic hepatitis C prognosis using transient elastography‐based liver stiffness: a systematic review and meta‐analysis. J Viral Hepat 2018;25:502‐513. [DOI] [PubMed] [Google Scholar]
- 18. Masuzaki R, Tateishi R, Yoshida H, Sato T, Ohki T, Goto T, et al. Assessing liver tumor stiffness by transient elastography. Hepatol Int 2007;1:394‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback C, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2019;17:630‐637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petta S, Sebastiani G, Vigano M, Ampuero J, Wong VW‐S, Boursier J, et al. Monitoring occurrence of liver‐related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin Gastroenterol Hepatol 2020. Jul 1:S1542‐3565(20)30908‐3 10.1016/j.cgh.2020.06.045. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21. Friedrich‐Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009;252:595‐604. [DOI] [PubMed] [Google Scholar]
- 22. Herrmann E, de Ledinghen V, Cassinotto C, Chu WC‐W, Leung VY‐F, Ferraioli G, et al. Assessment of biopsy‐proven liver fibrosis by two‐dimensional shear wave elastography: an individual patient data‐based meta‐analysis. Hepatology 2018;67:260‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Friedrich‐Rust M, Lupsor M, de Knegt R, Dries V, Buggisch P, Gebel M, et al. Point shear wave elastography by acoustic radiation force impulse quantification in comparison to transient elastography for the noninvasive assessment of liver fibrosis in chronic hepatitis C: a prospective international multicenter study. Ultraschall Med 2015;36:239‐247. [DOI] [PubMed] [Google Scholar]
- 24. Friedrich‐Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, et al. Acoustic radiation force impulse imaging for non‐invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat 2013;20:240‐247. [DOI] [PubMed] [Google Scholar]
- 25. Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598‐607. [DOI] [PubMed] [Google Scholar]
- 26. Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang X‐J, Zhan S‐H, et al. Performance of the aspartate aminotransferase‐to‐platelet ratio index for the staging of hepatitis C‐related fibrosis: an updated meta‐analysis. Hepatology 2011;53:726‐736. [DOI] [PubMed] [Google Scholar]
- 27. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 28. Heagerty PJ, Lumley T, Pepe MS. Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337‐344. [DOI] [PubMed] [Google Scholar]
- 29. European Association for the Study of the Liver . EASL‐ALEH clinical practice guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015;63:237‐264. [DOI] [PubMed] [Google Scholar]
- 30. Friedrich‐Rust M, Poynard T, Castera L. Critical comparison of elastography methods to assess chronic liver disease. Nat Rev Gastroenterol Hepatol 2016;13:402‐411. [DOI] [PubMed] [Google Scholar]
- 31. Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound‐based transient elastography for the detection of hepatic fibrosis: systematic review and meta‐analysis. Clin Gastroenterol Hepatol 2007;5:1214‐1220. [DOI] [PubMed] [Google Scholar]
- 32. Lee HW, Yoo EJ, Kim BK, Kim SU, Park JY, Kim DY, et al. Prediction of development of liver‐related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am J Gastroenterol 2014;109:1241‐1249. [DOI] [PubMed] [Google Scholar]
- 33. Lee HW, Chon YE, Kim SU, Kim BY, Park JY, Kim DY, et al. Predicting liver‐related events using transient elastography in chronic hepatitis C patients with sustained virological response. Gut Liv 2016;10:429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat 2007;14:360‐369. [DOI] [PubMed] [Google Scholar]
- 35. Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 2008;48:1718‐1723. [DOI] [PubMed] [Google Scholar]
- 36. Friedrich‐Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu‐Braticevici C, Strobel D, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta‐analysis. J Viral Hepat 2012;19:e212‐e219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1


