Figure 5.
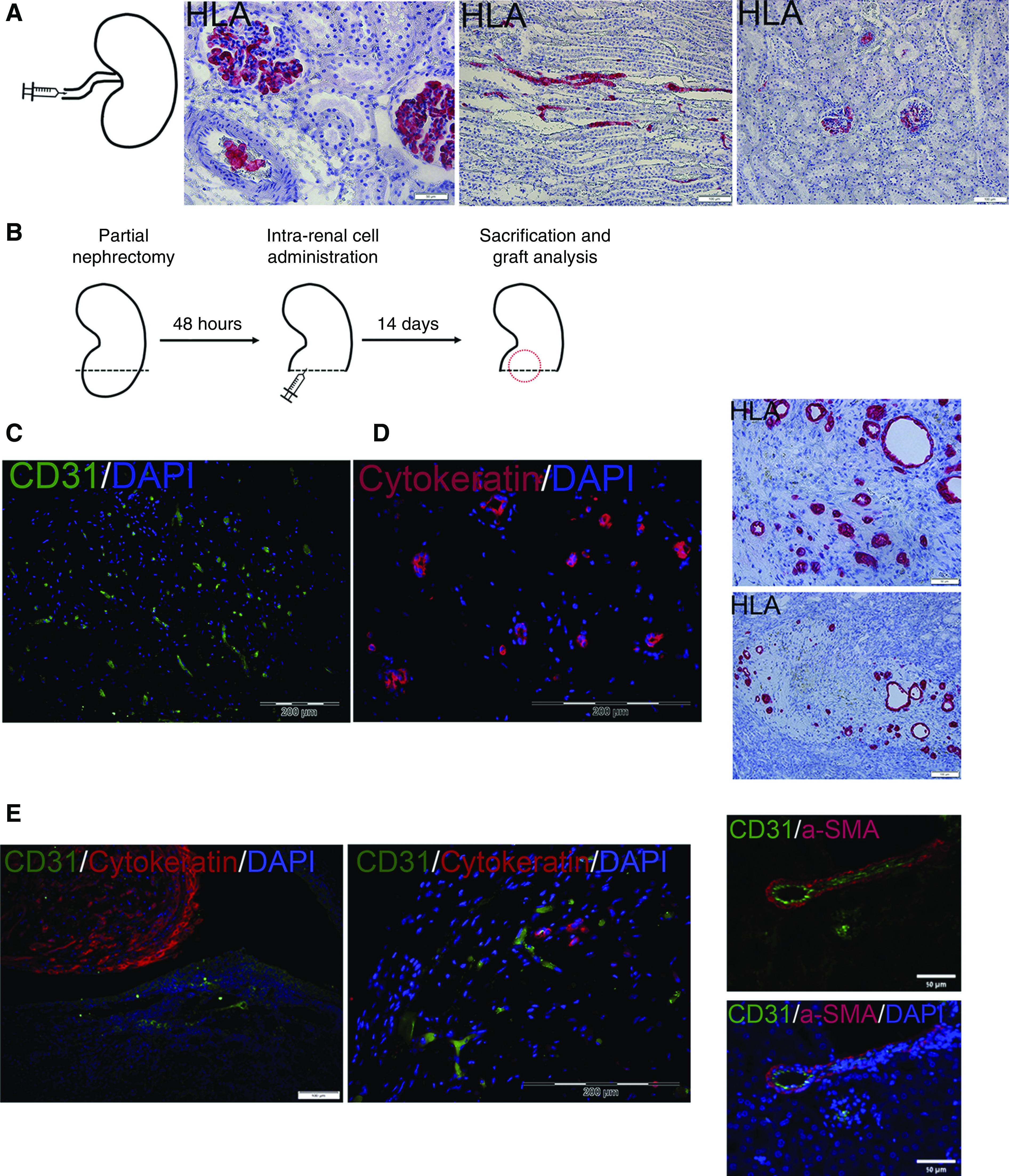
Coadministration of three-dimensional human nSPHs, MSCs, and ECFCs results in formation of renovascular units within damaged kidneys. (A) Initially, the intra-arterial route was tested (left, experimental scheme). Administration of donor nSPHs failed at localizing the cells to the renal parenchyma, as evident by the staining for HLA, which demonstrated the presence of human cells only within blood vessels and glomeruli. Scale bars: middle, 50 μm; right, 100 μm. (B) Experimental scheme: NOD-SCID mice underwent left partial nephrectomy. Forty eight hours later, they were divided into three groups: (1) intrarenal injection of MSCs and ECFCs; (2) intrarenal injection of human nSPHs; and (3) intrarenal injection of human nSPHs, MSCs, and ECFCs. All cells were injected within Matrigel into the renal parenchyma adjacent to the resection area. After 14 days, the mice were euthanized, and their kidneys were removed for analysis. (C) Grafts generated from injection of MSCs and ECFCs: immunofluorescence staining for human CD31 (green) demonstrates the presence of donor-derived blood vessels but no tubular structures. Scale bar, 200 μm. (D) Grafts generated from injection of nSPHs: immunofluorescence staining for cytokeratin (red, left) and IHC staining for HLA (red, right) demonstrated the presence of tubular structures but no blood vessels. Scale bars: top left, 50 μm; bottom left, 100 μm; right, 200 μm. (E) Grafts generated from injection of nSPHs, MSCs, and ECFCs. Left and middle: immunofluorescence staining for human CD31 (green) and cytokeratin (red), demonstrating the presence of both numerous tubular structures and donor-derived blood vessels; right: immunofluorescence staining for human CD31 (green) and the pericyte marker αSMA (red), demonstrating the formation of well developed donor-derived vessels, containing both endothelium and pericytes. Scale bars: left, 50 μm; middle, 200 μM; right, 50 μm.
