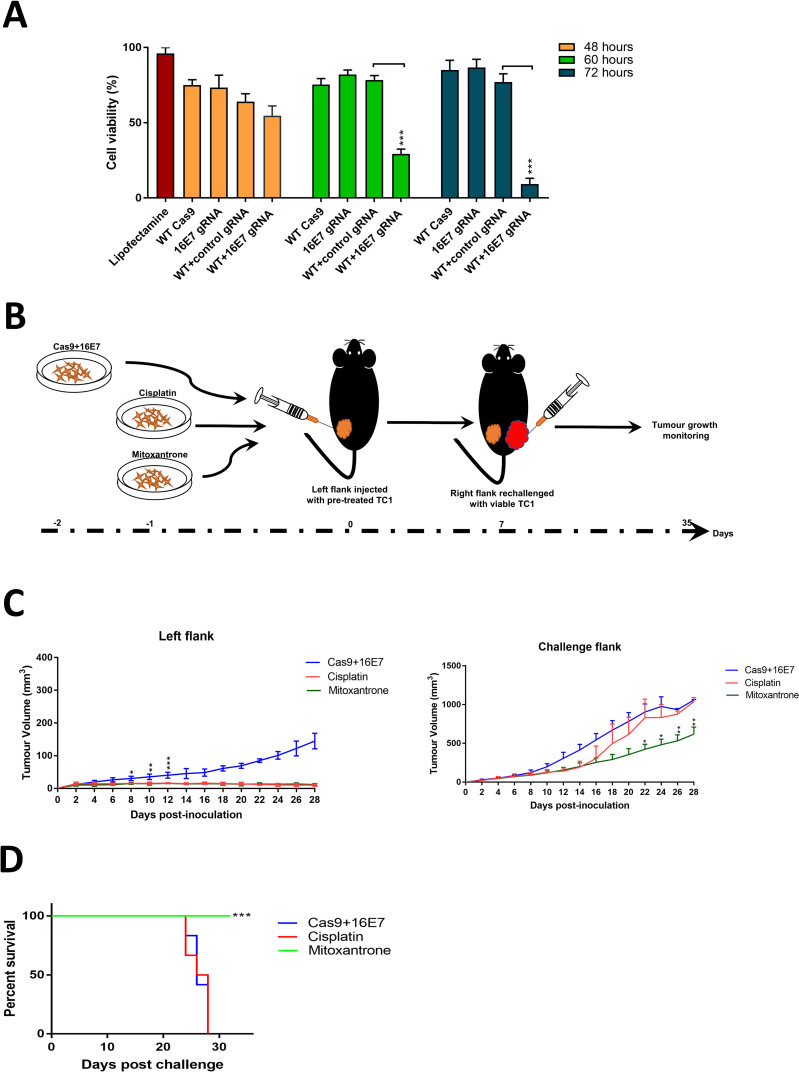
Fig 4. In vivo testing of the immunogenicity of cell death induced by CRISPR/Cas9 targeting HPV 16-driven tumours.
(A) Cell viability assay of HPV 16 +ve (TC1) cell line treated with Cas9 only, 16E7 gRNA only, Cas9+control gRNA or Cas9 +16E7 gRNA for 48, 60, or 72 hours before viability was assessed by MTT assay. (B) The experiment design and timeline to assess immunogenic cell death. TC1 cells were treated with either Cas9+16E7 (test group), cisplatin (non-ICD inducing, negative control), mitoxantrone (ICD inducing, positive control), injected in the left flank of C57BL/6J mice, and allowed to grow for seven days. The right flank was then rechallenged with viable (untreated) TC1 cells and followed up to the experiment endpoint (tumour volume of 1000 mm3). Tumour volume was assessed by digital caliper. (C) The tumour volume assessment of pre-treated TC1 cells (left flank) or the challenge viable cells (right flank) as explained in Fig 2A. (D) survival analysis of established TC1 xenografts after exposure to pre-treated TC1 cells with either 16E7, cisplatin, or mitoxantrone (right flank tumours). N = 5 per group. Data were represented as mean ± SD. Statistical significance was assessed by ANOVA with post-hoc analysis. * p<0.05, ** p<0.01, *** p<0.001.