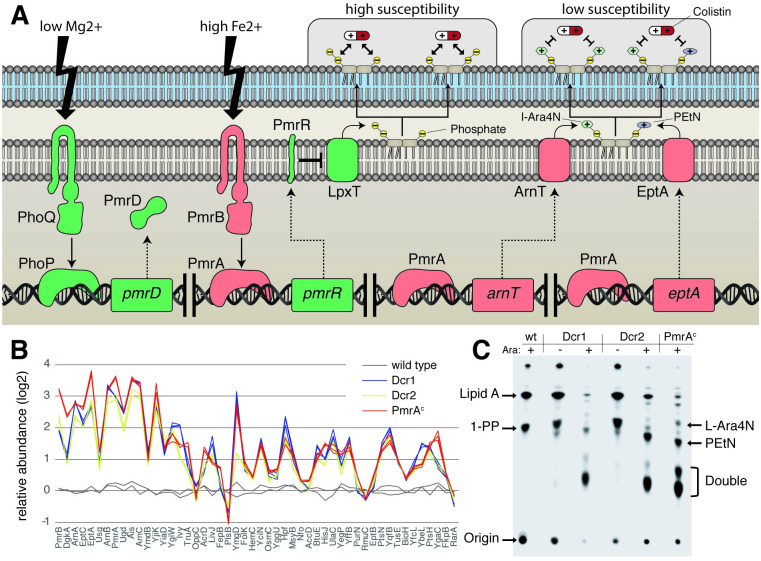
Fig 2. PmrAB-dependent lipid A modification.
(A) Schematic representation of important proteins involved in lipid A modification in E. coli BW25113. The negatively-charged terminal phosphate groups increase interaction with colistin whereas the positively-charged 4-amino-4-deoxy-l-arabinose (l-Ara4N) and phosphothanolamine (pEtN) modifications decrease susceptibility. The enzymes responsible for these modifications (ArnT and EptA) are under the control of the two-component system (TCS) PmrAB, which is activated by environmental signals as well as by the TCS PhoPQ via the connector protein PmrD. LpxT, a lipid A phosphotransferase under the control of PmrR, competes with EptA for lipid A modifications. PhoPQ, PmrD, PmrR and LpxT (green) could be removed without affecting the colistin resistance provided by the Dcr peptides, while removal of PmrAB, ArnT or EptA (red) resulted in a complete loss of function of the Dcr peptides. (B) Protein abundance of cells expressing Dcr 1 or 2 and a constitutive pmrA mutant compared to an empty vector control strain. The top 50 proteins with the most significant change in abundance (lowest p-value) in the pmrA mutant are shown. Measurements of the wild type, dcr1 and pmrA mutants were performed in triplicates and dcr2 in duplicate. Abundances are relative to the wild type replicate 1. (C) TLC-based separation of lipid A species isolated from wild-type E. coli BW25113 with empty control vector or expressing Dcr1 or 2 with or without induction via arabinose. A pmrA constitutive mutant served as a positive control for lipid A modifications.