Fig. 1.
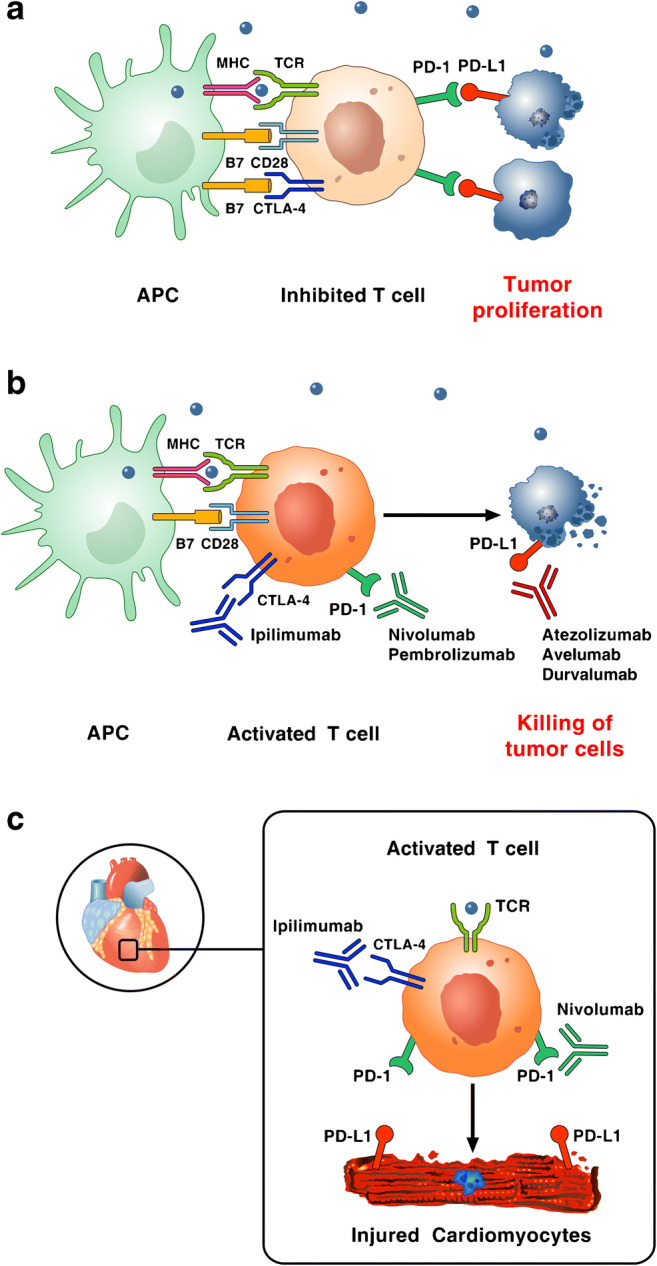
Reproduced with permission from [9]. a Tumor cells escape immune surveillance by promoting checkpoint activation. Tumor cells express the immune checkpoint activator PD-L1 and produce antigens (blue dots) that are captured by antigen presenting cells (APCs). These cells present antigens to cytotoxic CD8+ T cells through the interaction of major histocompatibility complex (MHC) molecules and T-cell receptor (TCR). T-cell activation requires co-stimulatory signals mediated by the interaction between B7 and CD28. Inhibitory signals from CTLA-4 and PD-1 checkpoints dampen T-cell response and promote tumor proliferation. b Checkpoint inhibitors stimulate T-cell activation. Monoclonal antibodies targeting CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab), and PD-L1 (atezolizumab, avelumab, durvalumab) block immune inhibitory checkpoints (CTLA-4, PD-1, and PD-L1, respectively) and restore anti-tumor immune response, resulting in tumor cell death via release of cytolytic molecules (e.g., TNF-α, Granzyme B, IFN-γ). c Hypothetical mechanism by which checkpoint inhibitors can promote autoimmune lymphocytic myocarditis. PD-L1 is expressed in human and murine cardiomyocytes and its expression can increase during myocardial injury. Combination of checkpoint blockade (ipilimumab plus nivolumab) unleashes immune responses and can cause autoimmune lymphocytic myocarditis. Importantly, lymphocytes in myocardium and tumors showed clonality of TCR suggesting that heart and tumors can share antigens (blue dot) recognized by the same T-cell clones
