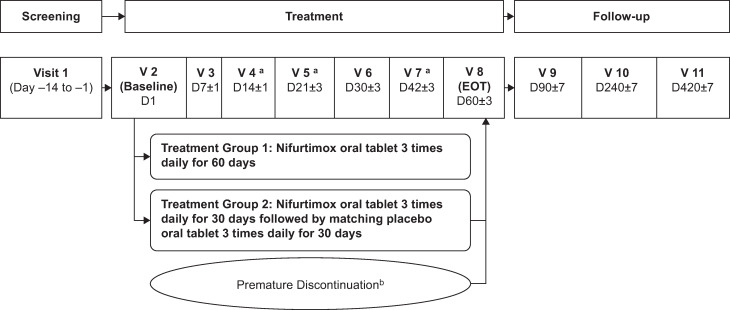
Fig 1. Schedule of study visits during treatment and follow-up.
D, day; EOT, end of treatment; V, visit. a At Visits 4, 5 and 7, the occurrence of adverse events, the use of concomitant medications, and treatment compliance were assessed via telephone. b Patients who discontinued treatment prematurely returned to the investigating centre for assessments at Visits 3, 6 and 8 (EOT), and underwent telephone assessments as described for Visits 4, 5 and 7. If patients were unable/unwilling to do so, they had to return to the investigational site 30 (±3) days after the last dose of study drug for EOT (Visit 8) assessments, and undergo telephone assessments as described for Visits 4, 5 and 7. If the patients was unable/unwilling to return to the clinic for the EOT Visit, then a telephone assessment as described for Visits 4, 5 and 7 was performed in lieu of Visit 8 assessments.