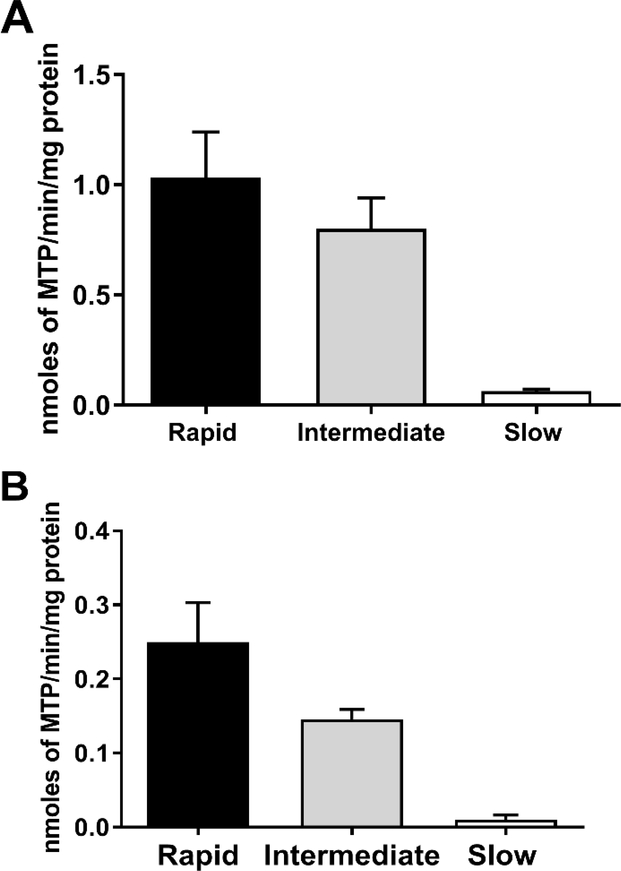
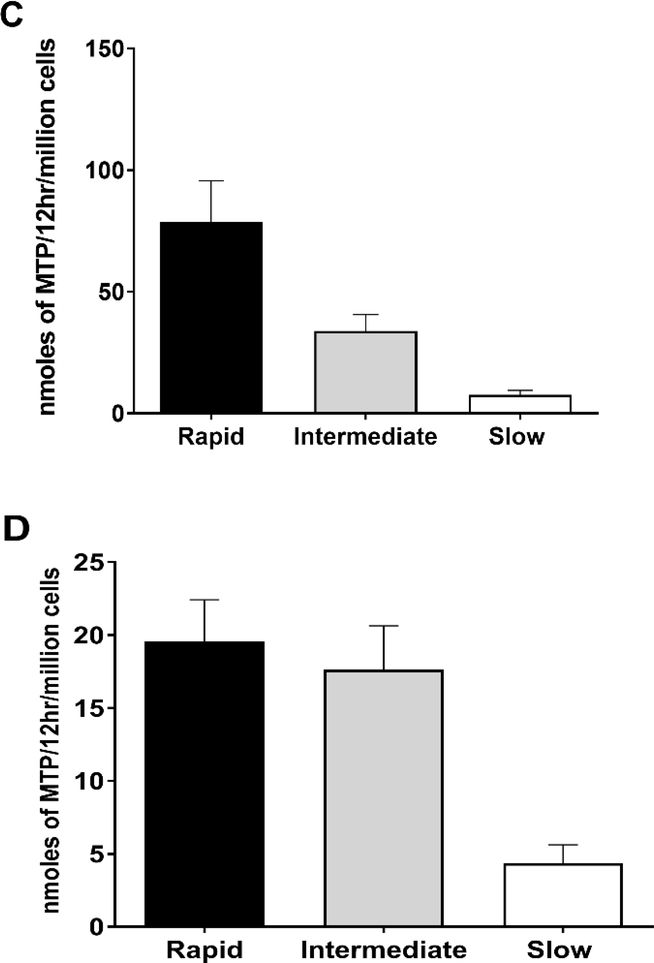
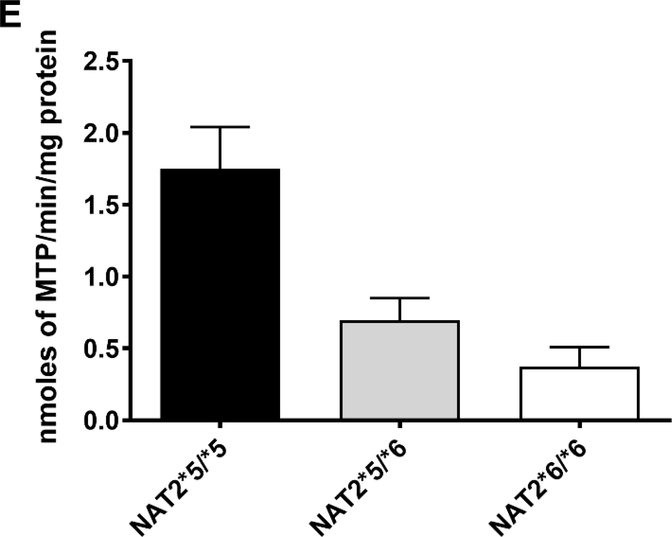
Figure 2.
HYD N-acetyltransferase activities in vitro in cryopreserved human hepatocytes from rapid, intermediate and slow NAT2 acetylators. Bars illustrate Mean ± SEM for HYD N-acetyltransferase activities from rapid (n=6), intermediate (n=5), and slow (n=5) NAT2 acetylators at 100 (A) or 10 (B) μM. HYD N-acetyltransferase activities differed significantly with respect to NAT2 phenotype at each concentration tested: 100 μM (p=0.002); and 10 μM hydralazine (p=0.0029). HYD N-Acetylation in situ in cryopreserved human hepatocytes from rapid, intermediate and slow acetylators. Bars illustrate Mean ± SEM HYD N-acetylation rates in rapid (solid bar; n=5), intermediate (gray bar; n=5) and slow (white bar; n=5) acetylators following incubation with 100 (C) or 10 (D) μM HYD. N-acetylation rates differed significantly among the rapid, intermediate and slow acetylators at 10 μM (p=0.002) and 100 μM (p=0.0015) HYD. (E): HYD N-acetylation in situ in cryopreserved human hepatocytes among slow NAT2 acetylator genotypes. Bars illustrate Mean ± SEM HYD N-acetylation rates in NAT2*5B/*5B (n=5), NAT2*5B/*6A (n=6), and NAT2*6A/*6A (n=5) genotypes. Hydralazine N-acetyltransferase activities differed significantly with respect to slow acetylator NAT2 genotype (p<0.001). Modified from [55].