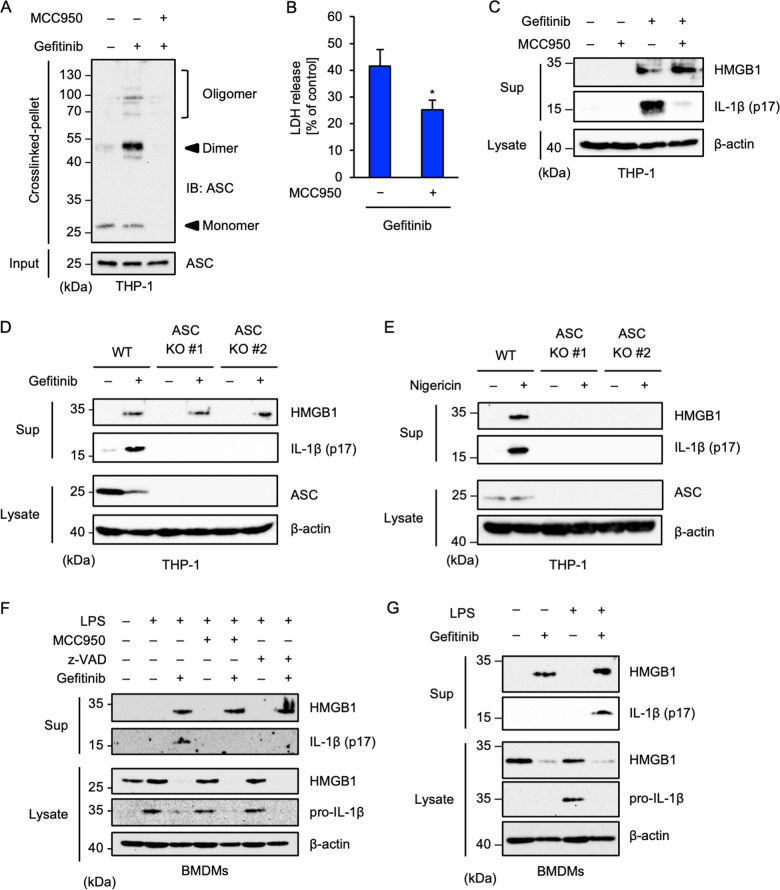
Fig. 5. Gefitinib promotes HMGB1 release independently of the NLRP3 inflammasome.
A, B, C The inhibitory effect of MCC950. PMA-differentiated THP-1 cells were pretreated with 1 μM MCC950 for 0.5 h and then treated with 20 μM gefitinib for 8 h. A DSS-mediated crosslinked pellets (Crosslinked-pellet) and soluble lysates (Input) were immunoblotted with anti-ASC antibody. B Cell cytotoxicity was measured by LDH release assay. Data shown are the mean ± S.D. Significant differences were determined by student’s t-test; *p < 0.05. C Cell-free supernatants (Sup) and cell lysates were subjected to immunoblotting with the indicated antibodies. D, E Requirement of ASC in gefitinib- or nigericin-induced HMGB1 release. PMA-differentiated WT and ASC KO THP-1 cells were treated with 20 μM gefitinib for 8 h or 5 μM nigericin for 2 h. Cell-free supernatants (Sup) and cell lysates were subjected to immunoblotting with the indicated antibodies. F The inhibitory effect of MCC950 or z-VAD on gefitinib-induced HMGB1 release. LPS-primed BMDMs were pretreated with 1 μM MCC950 or 20 µM z-VAD for 0.5 h and then treated with 20 μM gefitinib for 8 h. Cell-free supernatants (Sup) and cell lysates were subjected to immunoblotting with the indicated antibodies. G Requirement of LPS priming for gefitinib-induced HMGB1 release. LPS-primed or unprimed BMDMs were treated with 20 μM gefitinib for 8 h. Cell-free supernatants (Sup) and cell lysates were subjected to immunoblotting with indicated antibodies. All data in Fig. 5 are representatives of at least three independent experiments.