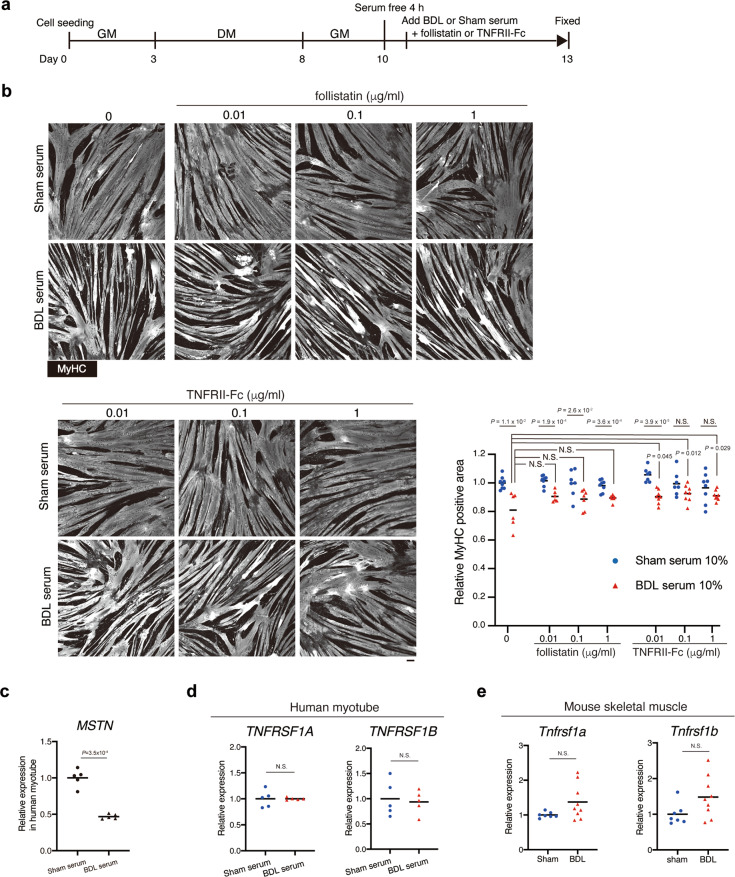
Fig. 4. TNFα is a causative substance of BDL mouse serum-induced myotube atrophy.
a Experimental design for examining the effects of MSTN or TNFα inhibition on mature human myotubes treated with BDL serum. b BDL or sham mouse serum-treated human myotubes were treated with follistatin or TNFRII-Fc at the concentrations indicated. Myotubes were stained for MyHC and the MyHC-positive area was quantified. c The expression levels of MSTN in the human myotubes treated with sham or BDL serum were quantified. d The expression levels of TNF receptor genes (TNFRSF1A and TNFRSF1B) in the human myotubes treated with sham or BDL serum were quantified. e The expression levels of TNF receptor genes (Tnfrsf1a and Tnfrsf1b) in TA muscles from sham or BDL operation mice were quantified. n = 8 (sham) and n = 6 (BDL) independent wells for control (no addition of follistatin or TNFRII-Fc), n = 7, 7, and 8 (sham) and n = 6, 7, and 7 (BDL) for 0.01, 0.1, and 1 μg/mL of follistatin, respectively. n = 7, 7, and 8 (sham) and n = 8, 8, and 8 (BDL) for 0.01, 0.1, and 1 μg/mL of TNFRII-Fc, respectively (b), n = 5 independent wells (c, d), n = 7 for sham and 9 for BDL mice (e). Data represent individual data points and the means. Data were analysed using the two-sided unpaired t-test, t-test with Welch’s correction, or ANOVA, followed by Dunnett’s post hoc test. NS not significant. Scale bar, 100 μm (b).