FIGURE 3.
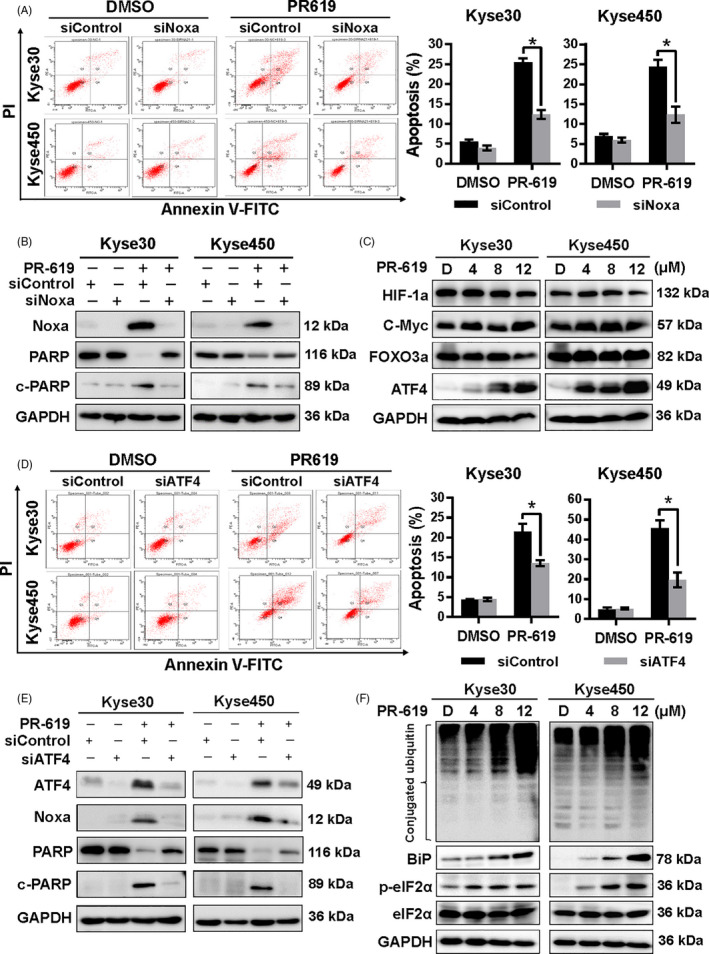
ATF4‐Noxa was responsible for PR‐619‐induced apoptosis in ESCC cells. A, Noxa was critical for apoptosis induced by PR‐619 in ESCC cells. After transfected with the control siRNA or Noxa siRNA, Kyse30 and Kyse450 cells were further treated with PR‐619 (8 μΜ) for 48 h. Apoptosis was quantified by Annexin V‐FITC/PI double‐staining analysis (left panel), and Annexin V+ cell populations were defined as apoptosis (right panel). B, Efficiency of siNoxa and its effect on the level of cleaved PARP. Kyse30 and Kyse450 cells were treated as described in panel A, and cell proteins were extracted for Western blotting analysis. GAPDH was used as the loading control. C, Screen of Noxa‐related transcription factors. Kyse30 and Kyse450 cells were treated with DMSO or PR‐619, and cell lysates were measured by Western blotting with specific antibodies. GAPDH was used as the loading control. D, The expression of ATF4 was responsible for PR‐619‐induced apoptosis in ESCC cells. After transfected with the control siRNA or ATF4 siRNA, Kyse30 and Kyse450 cells were further treated with PR‐619 (8 μΜ) for 48 h. Apoptosis was examined by Annexin V‐FITC/PI double‐staining analysis (left panel), and Annexin V+ cell populations were defined as apoptosis (right panel). E, Knockdown efficiency of siATF4 and its effect on the expression of Noxa and cleaved PARP were measured by Western blotting analysis. GAPDH was used as the loading control. F, PR‐619 treatment accumulated ubiquitinated proteins and activated ER stress. Kyse30 and Kyse450 cells were treated with PR‐619, and cell lysates were assessed by Western blotting with specific antibodies. GAPDH was used as the loading control. All data were representative of at least three independent experiments (n = 3; error bar, SD)
