Abstract
Purpose
The prognosis of young colorectal cancer (CRC) patients has not fully been addressed. The prognostic significance of systemic inflammatory markers was examined in those patients.
Methods
A total of 965 patients with resectable CRC were divided into young (≤ 50 years, n = 101) and old groups (> 51 years, n = 864). Neutrophil-to-lymphocyte ratio (NLR) > 5, derived NLR (dNLR) > 3, lymphocyte-to-monocyte ratio (LMR) < 2, platelet-to-lymphocyte ratio (PLR) > 150, and prognostic nutritional index (PNI) < 45 were analyzed for prognosis. Overall survival (OS) and progression-free survival (PFS) were compared using the log-rank test. A multivariate analysis was performed using a Cox proportional hazards regression model.
Results
In the young group, NLR > 5, LMR < 2, and PNI < 45 were significantly associated with OS with univariate analyses. dNLR > 3 and those markers showed significance for PFS. LMR < 2 was a significant marker for poor PFS (hazard ratio [HR], 5.81; P = 0.020) in the multivariate analysis. In the old group, all inflammatory markers were significantly associated with OS and PFS with univariate analyses. LMR < 2 (HR, 2.66; P = 0.016) and PNI < 45 (HR, 2.14; P = 0.016) were independently associated with OS in multivariate analyses. PLR > 150 (HR, 1.45; P = 0.036) and PNI < 45 (HR, 1.73; P = 0.002) were significant markers for PFS.
Conclusion
Systemic inflammation might be one of biologic factors that influence on prognosis of young CRC.
Keywords: Colorectal cancer, Prognosis, Systemic inflammatory markers, Young patients
INTRODUCTION
Colorectal cancer (CRC) is the third leading cause of cancer mortality worldwide [1]. Although the incidence of CRC in patients older than 50 years is decreasing, its steady increase has been reported in young patients [2,3]. Despite many studies on young-onset CRC, no consistent results have been reported on the prognosis and disease characteristics. Some studies have reported that the prognosis of young-onset CRC was poorer than that of old-onset CRC [4,5], while others have reported better survival for young patients [6,7]. Stage-stratified survival has been reported to be favorable for young patients with stage III and IV CRC [8]. Several clinical factors have been suggested to explain why young CRC patients have a better prognosis; better fitness, fewer comorbidities affecting surgical recovery, lower surgical mortality, and better tolerance of adjuvant therapy [9,10]. A higher prevalence of hereditary colon cancer such as Lynch syndrome has been suggested as another explanation for better survival [9]. The possibility that adverse clinicopathologic features of young-onset CRC might lead to an inferior prognosis has been suggested, but recent studies describing disease characteristics discrepant from overall survival (OS) have raised disease biology as a factor that might influence the prognosis of young patients [6,7,8].
Because chronic inflammation plays a substantial role in the development of various cancers [11], many previous studies have tried to clarify the link between cancer and systemic inflammation. In fact, a considerable body of evidence suggests that systemic inflammatory markers, represented by ratios of different WBCs or platelets, can predict the prognosis of various cancers, including CRC. The main immune cells related to cancer progression are neutrophils and monocytes such as macrophages [11,12]. Circulating neutrophils and monocytes giving rise to tumor-infiltrating macrophages are reported to be elevated along with increased myelopoiesis in cancer patients [12]. Platelets are involved in cancer progression through platelet-mediated recognition of cancer cells, which can lead to suppression of immune recognition and a subsequent failure to eradicate cancer cells [13]. In contrast to the role of the innate immune response, lymphocytes responsible for the adaptive immune system tend to exert anti-tumor effects through immunosurveillance [14]. A high neutrophil-to-lymphocyte ratio (NLR), one of the most concretely validated markers, is a poor prognostic factor in patients with perioperative and metastatic CRC [15,16]. Another inflammatory marker, the derived NLR (dNLR) has a prognostic value similar to that of the NLR [17,18]. The lymphocyte-to-monocyte ratio (LMR) has also been examined in CRC, and a low LMR is associated with poor prognosis [19,20]. Platelets are also useful in predicting the prognosis of CRC. A previous meta-analysis suggested that a high peripheral platelet-to-lymphocyte ratio (PLR) was significantly associated with poor OS in CRC [21].
Several studies have addressed the characteristics of young CRC patients, but prognostic factors for them have scarcely been investigated at all. Based on previous results, we thought that age might not be the only factor that determines prognosis of CRC patients. To look into the biologic factors that influence the prognosis of young CRC patients, we examined the effects of systemic inflammation on OS and progression-free survival (PFS) in young patients with resectable CRC.
METHODS
We retrospectively analyzed the data from 965 patients diagnosed with stage 0–III CRC at Korea University Guro Hospital between December 2003 and December 2017. The number of patients in ≤50 years (young patients) was 101, and the other 864 subjects (old patients) were >50 years. Patients treated with curative surgical or endoscopic resection, with or without adjuvant chemotherapy, were included in this study. Patients harboring primary cancers originating in ≥2 organs, with predisposing inflammatory bowel disease, or metastatic disease (including resectable liver or lung involvement) were excluded. This study was performed according to the principles of the Declaration of Helsinki, 1964, and the Institutional Review Board (IRB) of Korea University Guro Hospital approved the study protocol (No. 2018GR0242). Informed consent was waived by the IRB.
CRC diagnoses and histology classifications were made by well-experienced pathologists using surgically or endoscopically resected colorectal tissue. The stage of disease was determined according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual [22]. Tumors located proximal to the splenic flexure were regarded as right-sided, and tumors lodged from the descending colon to the rectum were left-sided CRC. The microsatellite instability (MSI) test was performed with PCR using a panel of 5 markers for microsatellites containing 2 mononucleotide repeats (BAT25 and BAT26) and 3 dinucleotide repeats (D5S346, D2S123, and D17S250). MSI-high (MSI-H) tumors were defined as having instability in 2 or more markers, and MSI-low (MSI-L) tumors were defined as having instability in 1 marker. Tumors with no instability were classified as microsatellite stable (MSS) tumors [23]. Tumors with deficient expression in 1 of 4 mismatch repair (MMR) proteins in immunohistochemical staining were also regarded as MSI-H without regard to the results of the MSI test. Patients harboring pathogenic mutations for 1 of 4 MMR genes in a germline mutation analysis and those who fulfilled the Amsterdam criteria were diagnosed with Lynch syndrome. A germline mutation for the APC gene was analyzed to diagnose familial adenomatous polyposis. Body mass index (BMI) was calculated as an individual's body mass (kg) divided by the square of height (m2). BMI ≥ 25 kg/m2 was defined as obese based on the Asia-Pacific criteria.
Differential WBC counts, platelet, and albumin measured at CRC diagnosis were collected for analyses. The systemic inflammatory markers analyzed were the NLR, LMR, PLR, dNLR, and prognostic nutritional index (PNI). The dNLR and PNI were calculated with the following equations, respectively: dNLR = absolute neutrophil count/WBC count-absolute neutrophil count [17], PNI = albumin (g/L) + (5 × total lymphocyte count × 103/µL) [24]. Cut-off levels for the systemic inflammatory markers were determined on the basis of previously reported thresholds: NLR > 5 [25], dNLR > 3 [17], LMR < 2, PLR > 150 [19], and PNI < 45 [24].
Patients were divided into 2 groups (young vs. old) to examine the association of systemic inflammatory markers with OS and PFS. The chi-square test and t-test were employed to compare clinical characteristics between young and old age groups. Differences in OS and PFS were analyzed using clinical parameters: age (>50 years); tumor stage; anatomical location of tumors (right vs. left); BMI (≥25 kg/m2); MSI (MSI-H vs. MSI-L and MSS); histologic features: differentiation of tumors (well-differentiated, moderately differentiated, poorly differentiated, mucinous adenocarcinoma, and unspecified differentiation); lymphatic invasion; perineural invasion; venous invasion of tumors; and the systemic inflammatory markers with cut-off levels described above. Initially recorded continuous laboratory variables were dichotomized for ease of statistical analysis. OS was defined as the period from the date of curative resection to the date of death from any cause. PFS was calculated from the date of curative resection to the date of documentation of progressive disease or death from any cause. The Kaplan-Meier method was used to estimate OS and PFS, and the log-rank test was used to compare OS and PFS according to the selected parameters. Significant risk factors found in log-rank test were included in multivariate analyses. A multivariate analysis was performed with a Cox proportional hazards regression model to address independent significant factors for OS and PFS. The results are described as hazard ratios (HRs) and 95% confidence intervals (CIs). P < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics ver. 20 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
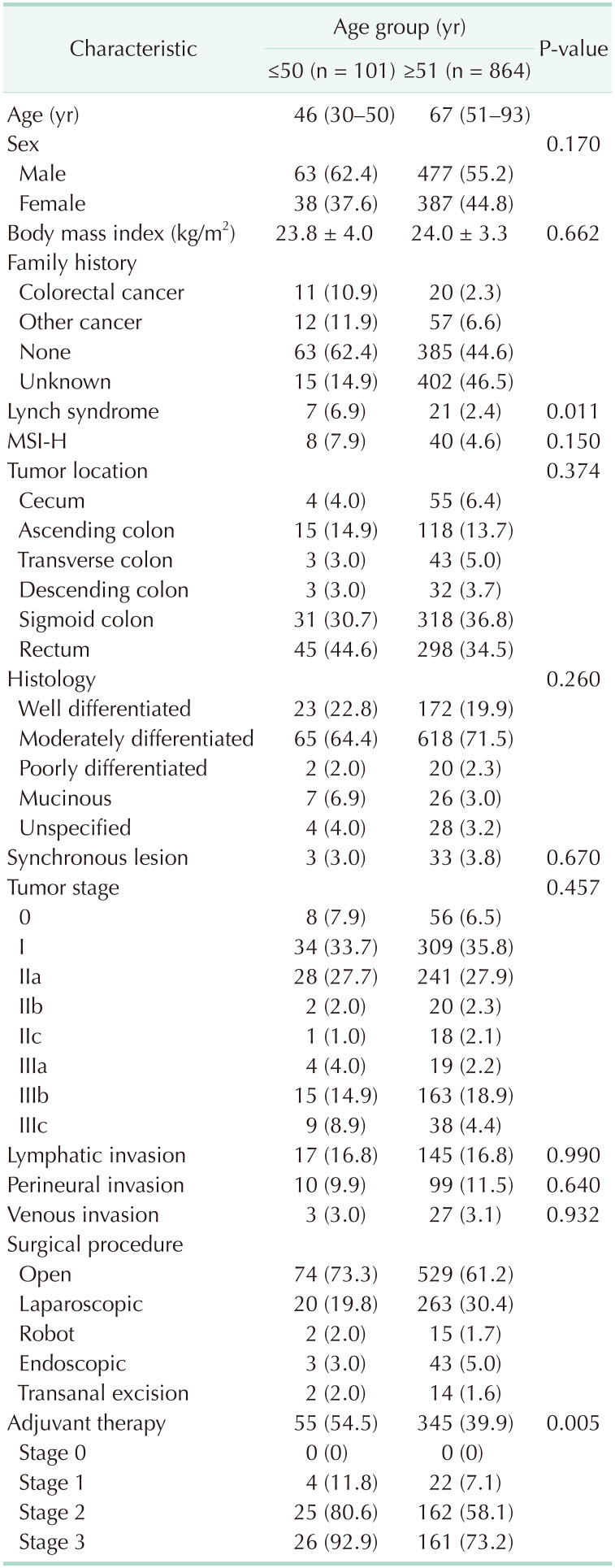
The characteristics of the 965 patients with resectable CRC are summarized in Table 1. The median age at diagnosis was 46 years (range, 30–50 years) and 67 years (range, 51–93 years) in the young and old groups, respectively. The number of patients with a family history of CRC in a first- or second-degree relative was 8 and 3, respectively, in the young group. In the old group, 18 of 20 patients had a family history of CRC in a first-degree relative. Tumors harboring MSI-H were found in 8 young and 40 old patients. Lynch syndrome was diagnosed in 21 (2.4%) and 7 patients (6.9%) in the old and young groups, respectively (P = 0.011). A germline mutation of the APC gene was documented in 2 young patients. Tumors were more frequently found in the left colon in both groups. Moderately differentiated CRC was the most common histology type in both age groups. The proportion of patients with synchronous CRC was comparable between the young (3.0%) and old groups (3.8%) (P = 0.670). Almost 1/3 of the patients in each group were diagnosed at stage I. Minimal differences were observed between the 2 groups in the proportion of patients with venous (3.0% in the young group and 3.1% in the old group, P = 0.932) and perineural (9.9% in the young group and 11.5% in the old group, P = 0.640) invasion. Lymphatic invasion was documented in 16.8% of the patients in each age group (P = 0.990). Young patients (54.5%) received adjuvant therapy more frequently than old patients (39.9%) did (P = 0.005). Patients in stage I received adjuvant therapy for the following reasons; lymphatic, venous, perineural invasion of tumors, physicians' concern, or a patient wanted to receive the adjuvant therapy.
Table 1. Characteristics of patients with colorectal cancer (n = 965).
Values are presented as median (range), number (%), or mean ± standard deviation.
MSI-H, microsatellite instability-high.
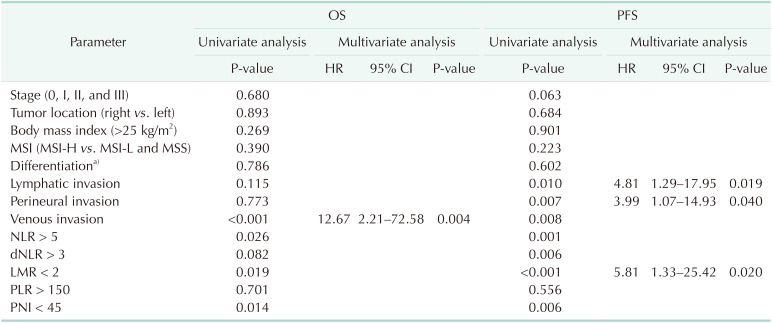
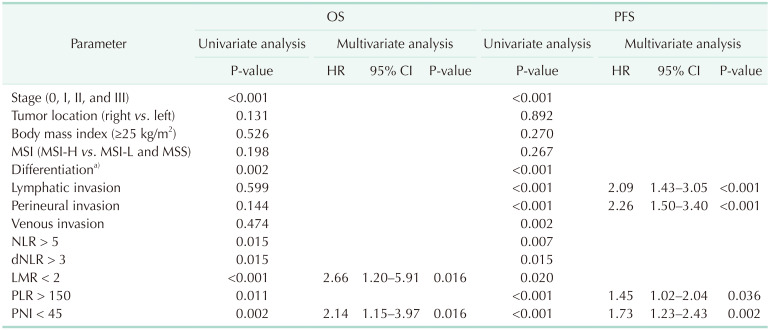
Clinical parameters (age, cancer stage, location, BMI, and MSI status), histologic features (differentiation of tumors and lymphatic/perineural/venous invasion) and systemic inflammatory markers were evaluated on the association with the prognosis of patients with resectable CRC. Six-year OS was 88.6% (95% CI, 80.4%–96.8%) with a median follow-up of 101.5 months in the young group. No clinical parameters were associated with OS or PFS in the univariate analyses. Venous invasion of tumors was significantly associated with OS (P < 0.001). Among the systemic inflammatory markers, NLR > 5, LMR < 2, and PNI < 45 were significantly associated with OS (P < 0.05). The multivariate analysis found no inflammatory markers or clinical parameters independently significantly associated with OS. Among histologic features, venous invasion was independently, significantly associated with OS (HR, 12.67; 95% CI, 2.21–72.58; P = 0.004). Six-year PFS was 82.2% (95% CI, 73.4%–91.0%) in the young group. Lymphatic (P = 0.010), perineural (P = 0.007), and venous (P = 0.008) invasion were significantly associated with PFS in the univariate analyses. NLR > 5, dNLR > 3, LMR < 2, and PNI < 45 were significant systemic inflammatory markers associated with PFS (P < 0.05). Lymphatic invasion (HR, 4.81; 95% CI, 1.29–17.95; P = 0.019) and perineural invasion (HR, 3.99; 95% CI, 1.07–14.93; P = 0.040) were independently, significantly associated with PFS in the multivariate analyses. LMR < 2 remained a significant prognostic factor among systemic inflammatory markers for poor PFS (HR, 5.81; 95% CI, 1.33–25.42; P = 0.020) (Table 2). In the old group, 6-year OS and PFS were 93.1% (95% CI, 90.9%–95.3%) and 80.0% (95% CI, 76.9%–83.1%), respectively, with a median follow-up of 84.9 months. AJCC cancer stage, differentiation of tumors, and all inflammatory markers (NLR > 5, dNLR > 3, LMR < 2, PLR > 150, and PNI < 45) were significantly associated with OS and PFS (P < 0.05) in the univariate analyses. Lymphatic, perineural, and venous invasion of tumors were significantly associated with PFS (P < 0.05). The multivariate analysis revealed that LMR < 2 (HR, 2.66; 95% CI, 1.20–5.91; P = 0.016) and PNI < 45 (HR, 2.14; 95% CI, 1.15–3.97; P = 0.016) were independently, significantly associated with OS. Lymphatic invasion (HR, 2.09; 95% CI, 1.43–3.05; P < 0.001) and perineural invasion (HR, 2.26; 95% CI, 1.50–3.40; P < 0.001) were independently, significantly associated with PFS. PLR > 150 (HR, 1.45; 95% CI, 1.02–2.04; P = 0.036) and PNI < 45 (HR, 1.73; 95% CI, 1.23–2.43; P = 0.002) were significant inflammatory markers for PFS (Table 3).
Table 2. Prognostic factors for OS and PFS in the young group (n = 101).
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; MSI-H, MSI-high; MSI-L, MSI-low; MSS, microsatellite stable; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived NLR; LMR, lymphocyte-tomonocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index.
a)Well, moderately, poor, mucinous, and unspecified.
Table 3. Prognostic factors for OS and PFS in the old group (n = 864).
OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; MSI, microsatellite instability; MSI-H, MSI-high; MSI-L, MSI-low; MSS, microsatellite stable; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived NLR; LMR, lymphocyte-tomonocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index.
a)Well, moderately, poor, mucinous, and unspecified.
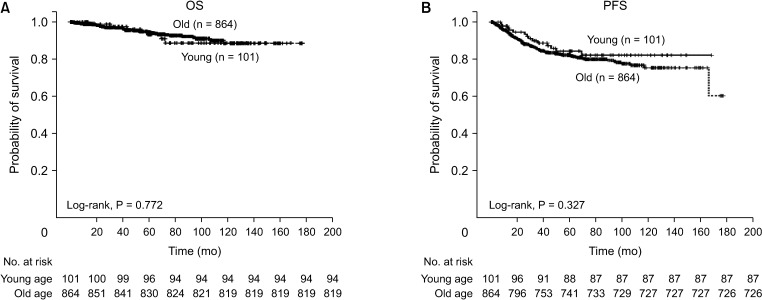
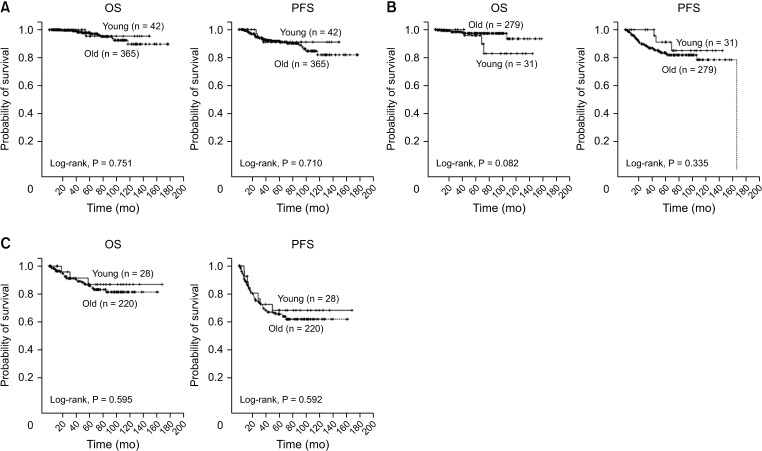
A comparison of OS and PFS between young and old patients was performed for the controversial prognosis of young patients with CRC. We observed no significant difference in OS (P = 0.772, Fig. 1A) or PFS (P = 0.327, Fig. 1B) between the 2 age groups. Our analysis of the stage-stratified survival of patients with stage 0–I (Fig. 2A), II (Fig. 2B), and III (Fig. 2C) CRC also showed no significant difference in OS or PFS according to age. We also looked for differences in OS or PFS according to the age of patients with under or beyond the cut-off values of inflammatory markers (Supplementary Fig. 1). In patients with an NLR ≤ 5, we found no significant differences in OS or PFS according to age (Supplementary Fig. 1A). OS and PFS between the 2 age groups did not differ significantly in patients with NLR > 5, either (Supplementary Fig. 1B). Among the patient groups with dNLR > 3 (Supplementary Fig. 1C) or dNLR ≤ 3 (Supplementary Fig. 1D), LMR < 2 (Supplementary Fig. 1E) or LMR ≥ 2 (Supplementary Fig. 1F), PLR >150 (Supplementary Fig. 1G) or PLR ≤ 150 (Supplementary Fig. 1H), and PNI < 45 (Supplementary Fig. 1I) or PNI ≥ 45 (Supplementary Fig. 1J), age difference did not significantly affect prognosis in terms of OS or PFS.
Fig. 1. (A) Overall survival (OS) and (B) progression-free survival (PFS) of colorectal cancer patients. OS and PFS did not differ significantly between the young (≤50 years) and old (>50 years) groups.
Fig. 2. Stage-stratified survival of patients with colorectal cancer (CRC). (A) No significant difference in overall survival (OS, left) or progression-free survival (PFS, right) between the young (≤50 years) and old patients (>50 years) with stage 0 and I CRC. (B) OS (left) and PFS (right) of stage II CRC patients did not differ significantly according to age. (C) Comparable OS (left) and PFS (right) in stage III CRC patients regardless of age.
DISCUSSION
Our results show that LMR usefully predict the prognosis of patients with resectable CRC younger than 50 years. The crucial role of systemic inflammation in tumorigenesis has been discussed for many years [11]. The inflammatory markers examined are ratios of immune cells involved in innate and adaptive immunity. Our results suggest that systemic inflammatory markers play a consistent, significant role in predicting the prognosis of young patients with resectable CRC. Intriguingly, no clinical parameters, including MSI-H, were associated with OS or PFS with multivariate analyses in both age groups. A germline mutation in an MMR gene results in a high mutation burden leading to the generation of numerous neoantigens, which subsequently activates the adaptive immune system. Therefore, MSI-H and a diagnosis of Lynch syndrome have been regarded as parameters indicating a favorable prognosis, but we found no significant difference in OS or PFS between patients with tumors harboring MSS and those with MSI-H tumors in both age groups. This could be explained by the small number of patients with MSI-H, considering a nonsignificant tendency toward improved survival in patients with MSI-H. Another possible explanation is that the ratio of immune cells involved in innate and adaptive immunity might be superior to MSI status in predicting the prognosis of resectable CRC. As a matter of fact, van Herk et al. [26] investigated the immunophenotypes of early-onset CRC, specifically the expression of human leukocyte antigen (HLA) class I and programmed death-ligand 1, and found that reduced expression of HLA class I was associated with liver metastases, suggesting not only an increased mutation burden but also the activation of lymphocytes by antigen presentation with retained HLA-I function is important in activating adaptive immunity in young patients.
LMR < 2 was an independent prognostic factor for poor PFS in the young in this study. A recent study reported that higher preoperative lymphocyte blood count to WBC count ratio correlated with longer survival in CRC patients younger than 50 years, consistent with our results [27]. Intriguingly, different association of LMR < 2 with PFS or OS in young and old group, respectively, was observed in multivariate analyses. This could be attributed to functional heterogeneity of monocyte according to different environmental stimuli exerting pro- or anticancer immunity [28]. A recent study on immunohistochemical profile of macrophage revealed proinflammatory macrophages expressing CD 80 are mainly located at tumor-adjacent normal mucosa in CRC. They were also reported to have protective role from risk for relapse. On the other hand, the lower ratio of CD 80 and CD 163 macrophage, functioning as anti-inflammatory macrophages, was also reported to be related to poor prognosis in stage III CRC [29]. Therefore, it would be compelling to investigate if there is involvement of different fraction of macrophage with different function according to age groups. In addition, substantial numbers of patients in our study were lost to follow-up due to the favorable prognosis of patients with resectable CRC, giving rise to censored data in our survival analyses. This, along with the small numbers of patients analyzed, could have contributed to our finding that no systemic inflammatory markers are independently significant for OS in the young group, one of the limitations in this study.
On the other hand, PNI < 45 was a significant inflammatory marker for OS and PFS only in the old age group, but not in the young age group. PNI usually reflects nutritional and immunologic status of patients. A previous study reported PNI values had negative correlation with age, and the mean age of patients with low-PNI values was significantly higher than that of patients with high-PNI values [30]. In this study, we also found significantly larger proportion in the old age group was PNI < 45 (44.3%), comparing to proportion of young patients with PNI < 45 (7.9%, P < 0.01). Therefore, we thought relatively poorer nutritional status of old patients than that of young patients could have contributed to significant association of PNI < 45 with prognosis of old patients.
Although no consistent data on the prognosis of young CRC patients have been reported, several recent studies have shown improved outcomes in young patients than in older patients despite aggressive disease features, suggesting that adverse clinicopathologic features are not the major determinants of prognosis in young CRC patients [7,8,27]. Our study found no differences between the 2 age groups in OS or PFS, including an analysis of stage-stratified survival. In addition, patients within the same range of values of each inflammatory marker did not show difference in OS or PFS according to ages despite clear association of inflammatory markers with survival, suggesting biologic factors involved in tumorigenesis rather than age itself might be more important in prognosis of patients with resectable CRC.
Our findings provide valuable new information about biologic factors other than clinicopathologic characteristics and their effects on the prognosis of young patients with resectable CRC. As the number of young CRC patients increases, efforts to discern who benefit from treatment have focused on evaluation of prognosis. However, no obvious prognostic factors affecting the survival of young CRC patients have been found. The inconsistent thresholds for inflammatory markers used in previous studies have often caused issues in applying research data in the clinical setting to differentiate patients with a poor prognosis from those with a positive prognosis. The thresholds used for each inflammatory marker in this study were taken from previous studies [17,19,24,25], which might help clinicians choose optimal treatments for young patients.
In summary, LMR < 2 was significantly, independently associated with PFS in young patients with resectable CRC. In the old group, LMR < 2 and PNI < 45 were independently, significantly associated with OS. PLR > 150 and PNI < 45 were significant inflammatory markers for PFS. However, no clinical parameters including age were significantly associated with prognosis. Considering above results, systemic inflammation might be one of potential factors which consistently affects on prognosis of both young and old patients with resectable CRC.
Footnotes
This study was presented at the International Society for Gastrointestinal Hereditary Tumours (InSiGHT) 2019 Meeting in Auckland on 21 March, 2019.
Fund/Grant Support: This paper was supported by Wonkwang University in 2020.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: SL, SIL.
- Formal Analysis: SL.
- Investigation: All authors.
- Methodology: SL.
- Project Administration: SL.
- Writing — Original Draft: SL.
- Writing — Review & Editing: All authors.
SUPPLEMENTARY MATERIALS
Supplementary Fig. 1 can be found via https://doi.org/10.4174/astr.2021.100.1.25.
Overall survival (OS) and progression-free survival (PFS) according to age in each following group. No significant difference in OS or PFS according to age in each of the following group of patients. (A) neutrophil-to-lymphocyte ratio (NLR) ≤ 5, (B) NLR > 5, (C) derived NLR (dNLR) > 3, (D) dNLR ≤ 3, (E) lymphocyte-to-monocyte ratio (LMR) < 2, (F) LMR ≥ 2, (G) platelet-to-lymphocyte ratio (PLR) > 150, (H) PLR ≤ 150, (I) prognostic nutritional index (PNI) < 45, and (J) PNI ≥ 45.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Austin H, Henley SJ, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;25:191–201. doi: 10.1007/s10552-013-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connell JB, Maggard MA, Livingston EH, Yo CK. Colorectal cancer in the young. Am J Surg. 2004;187:343–348. doi: 10.1016/j.amjsurg.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Khan SA, Morris M, Idrees K, Gimbel MI, Rosenberg S, Zeng Z, et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg. 2016;51:1812–1817. doi: 10.1016/j.jpedsurg.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quah HM, Joseph R, Schrag D, Shia J, Guillem JG, Paty PB, et al. Young age influences treatment but not outcome of colon cancer. Ann Surg Oncol. 2007;14:2759–2765. doi: 10.1245/s10434-007-9465-x. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez L, Brennan K, Karim S, Nanji S, Patel SV, Booth CM. Disease characteristics, clinical management, and outcomes of young patients with colon cancer: a population-based study. Clin Colorectal Cancer. 2018;17:e651–e661. doi: 10.1016/j.clcc.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–1139. doi: 10.1038/modpathol.2012.61. [DOI] [PubMed] [Google Scholar]
- 9.Cavestro GM, Mannucci A, Zuppardo RA, Di Leo M, Stoffel E, Tonon G. Early onset sporadic colorectal cancer: worrisome trends and oncogenic features. Dig Liver Dis. 2018;50:521–532. doi: 10.1016/j.dld.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Liang JT, Huang KC, Cheng AL, Jeng YM, Wu MS, Wang SM. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br J Surg. 2003;90:205–214. doi: 10.1002/bjs.4015. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 12.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 13.Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231–269. doi: 10.1007/s10555-014-9498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rashtak S, Ruan X, Druliner BR, Liu H, Therneau T, Mouchli M, et al. Peripheral neutrophil to lymphocyte ratio improves prognostication in colon cancer. Clin Colorectal Cancer. 2017;16:115–123. doi: 10.1016/j.clcc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riedl JM, Posch F, Moik F, Bezan A, Szkandera J, Smolle MA, et al. Inflammatory biomarkers in metastatic colorectal cancer: prognost ic and predictive role beyond the first line setting. Oncotarget. 2017;8:96048–96061. doi: 10.18632/oncotarget.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109:395–400. doi: 10.1038/bjc.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolan RD, McSorley ST, Park JH, Watt DG, Roxburgh CS, Horgan PG, et al. The prognost ic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119:40–51. doi: 10.1038/s41416-018-0095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stotz M, Pichler M, Absenger G, Szkandera J, Arminger F, Schaberl-Moser R, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer. 2014;110:435–440. doi: 10.1038/bjc.2013.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan D, Fu Y, Su Q, Wang H. Prognostic role of platelet-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3837. doi: 10.1097/MD.0000000000003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454–1455. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- 23.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 24.Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–2692. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 26.van Herk ME, Lee M, Rytterdahl M, Kop ME, Ramalheiro AF, van der Breggen R, et al. Abstract 2942: Immunophenotypes in young-onset colorectal cancer. Cancer Res. 2017;77(13 Suppl):2942. [Google Scholar]
- 27.Fiorot A, Pozza A, Ruffolo C, Caratozzolo E, Bonariol L, D'Amico FE, et al. Colorectal cancer in the young: a possible role for immune surveillance. Acta Chir Belg. 2018;118:7–14. doi: 10.1080/00015458.2017.1353233. [DOI] [PubMed] [Google Scholar]
- 28.Cortese N, Soldani C, Franceschini B, Barbagallo M, Marchesi F, Torzilli G, et al. Macrophages in colorectal cancer liver metastases. Cancers (Basel) 2019;11:633. doi: 10.3390/cancers11050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto ML, Rios E, Durães C, Ribeiro R, Machado JC, Mantovani A, et al. The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer. Front Immunol. 2019;10:1875. doi: 10.3389/fimmu.2019.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, et al. Prognostic nutritional index predicts severe compl icat ions, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58:1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overall survival (OS) and progression-free survival (PFS) according to age in each following group. No significant difference in OS or PFS according to age in each of the following group of patients. (A) neutrophil-to-lymphocyte ratio (NLR) ≤ 5, (B) NLR > 5, (C) derived NLR (dNLR) > 3, (D) dNLR ≤ 3, (E) lymphocyte-to-monocyte ratio (LMR) < 2, (F) LMR ≥ 2, (G) platelet-to-lymphocyte ratio (PLR) > 150, (H) PLR ≤ 150, (I) prognostic nutritional index (PNI) < 45, and (J) PNI ≥ 45.