Abstract
Rationale & Objective
Although coronavirus disease 2019 (COVID-19) has been associated with acute kidney injury (AKI), it is unclear whether this association is independent of traditional risk factors such as hypotension, nephrotoxin exposure, and inflammation. We tested the independent association of COVID-19 with AKI.
Study Design
Multicenter, observational, cohort study.
Setting & Participants
Patients admitted to 1 of 6 hospitals within the Yale New Haven Health System between March 10, 2020, and August 31, 2020, with results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing via polymerase chain reaction of a nasopharyngeal sample.
Exposure
Positive test for SARS-CoV-2.
Outcome
AKI by KDIGO (Kidney Disease: Improving Global Outcomes) criteria.
Analytical Approach
Evaluated the association of COVID-19 with AKI after controlling for time-invariant factors at admission (eg, demographic characteristics, comorbidities) and time-varying factors updated continuously during hospitalization (eg, vital signs, medications, laboratory results, respiratory failure) using time-updated Cox proportional hazard models.
Results
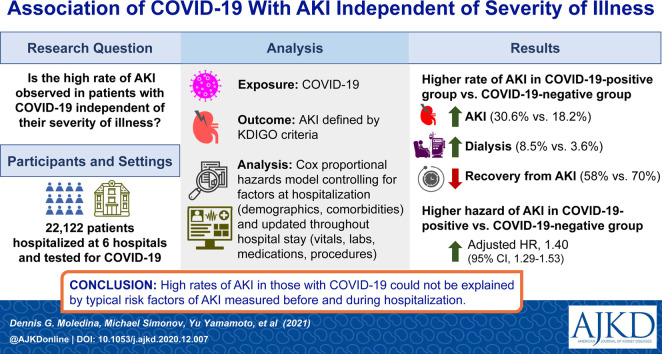
Of the 22,122 patients hospitalized, 2,600 tested positive and 19,522 tested negative for SARS-CoV-2. Compared with patients who tested negative, patients with COVID-19 had more AKI (30.6% vs 18.2%; absolute risk difference, 12.5% [95% CI, 10.6%-14.3%]) and dialysis-requiring AKI (8.5% vs 3.6%) and lower rates of recovery from AKI (58% vs 69.8%). Compared with patients without COVID-19, patients with COVID-19 had higher inflammatory marker levels (C-reactive protein, ferritin) and greater use of vasopressors and diuretic agents. Compared with patients without COVID-19, patients with COVID-19 had a higher rate of AKI in univariable analysis (hazard ratio, 1.84 [95% CI, 1.73-1.95]). In a fully adjusted model controlling for demographic variables, comorbidities, vital signs, medications, and laboratory results, COVID-19 remained associated with a high rate of AKI (adjusted hazard ratio, 1.40 [95% CI, 1.29-1.53]).
Limitations
Possibility of residual confounding.
Conclusions
COVID-19 is associated with high rates of AKI not fully explained by adjustment for known risk factors. This suggests the presence of mechanisms of AKI not accounted for in this analysis, which may include a direct effect of COVID-19 on the kidney or other unmeasured mediators. Future studies should evaluate the possible unique pathways by which COVID-19 may cause AKI.
Index Words: Coronavirus disease 2019 (COVID-19), acute kidney injury (AKI), risk factors, inflammation, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), hospital-acquired AKI, hypotension, C-reactive protein (CRP), ferritin, serum creatinine, dialysis, death, mortality, renal recovery, time-updated variables
Graphical abstract
Plain-Language Summary.
One third of patients hospitalized with coronavirus disease 2019 (COVID-19) experience acute kidney injury (AKI), which is more than in other hospitalized patients. Patients with COVID-19 carry many well known risk factors for AKI, including severe lung disease requiring mechanical ventilation, shock, and significant inflammation. Whether higher rates of AKI in COVID-19 are greater than what could be expected in patients with similar risk factors is unknown. We compared AKI rates between those with and without COVID-19 after controlling for risk factors for AKI before and during hospitalization. We found that COVID-19 was independently associated with high rates of AKI. This indicates that some of the AKI risk in patients with COVID-19 is unexplained by traditional AKI risk factors and is unique to this disease.
Coronavirus disease 2019 (COVID-19), the pandemic illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected more than 107 million individuals and caused more than 2.3 million deaths as of February 2021. Initially considered a respiratory illness, COVID-19 is now recognized to affect multiple organ systems, including lungs, heart, brain, endothelium, and kidneys. Acute kidney injury (AKI) was reported in 46%-57% of patients with COVID-19 hospitalized at tertiary-care hospitals in New York during the first phase of the pandemic and in 32%-37% in subsequent reports.1, 2, 3, 4 Such a high rate of AKI in patients with COVID-19 has stressed health care systems, including provision of dialysis,5 , 6 and may be associated with long-term patient harm.7
Whether the occurrence of AKI in patients with COVID-19 is out of proportion to that which could be expected in patients with similar degrees of illness is unclear. Patients with COVID-19 could experience higher rates of AKI due to the direct effects of the virus on the kidneys8 or the accompanying inflammatory response, or as a result of higher occurrence of AKI risk factors such as hemodynamic injury or nephrotoxin exposure. Although studies have reported possible direct infection of the kidneys9, 10, 11, 12 and glomerular disease,13, 14, 15, 16 the predominant histologic lesion reported is acute tubular injury.17 , 18 This indicates that AKI in COVID-19 is more likely to occur via pathways unrelated to direct kidney infection such as acute respiratory distress syndrome requiring mechanical ventilation and diuretic agents, hypotension, nephrotoxin exposure, or severe inflammatory response. Although studies have reported higher occurrence of AKI in patients with COVID-19,3 whether the higher rate of AKI in patients with COVID-19 is out of proportion with the occurrence of the aforementioned AKI risk factors and exposures during hospitalization is currently unknown.
In this multicenter, observational cohort study, we tested the hypothesis that AKI in patients with COVID-19 would be driven by similar risk factors as AKI in patients without COVID-19. We compared the incidence of AKI in patients with and without COVID-19 overall and after controlling for quantifiable, clinically validated risk factors of AKI, including time-invariant factors (eg, demographic data and comorbidities) as well as time-updated indicators of disease severity (eg, vital signs, laboratory results, use of medications, and need for mechanical ventilation).
Methods
Patients and Settings
We included patients with complete hospitalizations (admitted and discharged or died) at any one of 6 hospitals within the Yale New Haven Health System between March 10, 2020, and August 31, 2020, who received a diagnostic test for SARS-CoV-2 within 7 days before or 48 hours after admission. Beginning April 24, 2020, testing was performed for all admitted patients. Before this date, testing was limited to patients with febrile illness with respiratory derangements as well as travel/contact history. The Yale New Haven Health System includes 6 hospitals in southern Connecticut and Rhode Island with a mix of teaching and nonteaching, urban and suburban, and university and community hospitals.19 , 20 We excluded patients < 18 years of age, those with end-stage kidney disease or kidney transplant (International Classification of Diseases, Tenth Revision, codes N18.6 and Z94, respectively) in a previous encounter, and those with a first-encounter serum creatinine (Scr) level ≥ 4 mg/dL. We included only the first hospital encounter during the observation period. We did not collect data on patients who opted out of research participation. This study was approved by the Yale Human Investigation Committee (no. 2000027733) and operated under a waiver of informed consent as minimal-risk medical record research.
Exposure and Outcomes
Exposure of interest was a positive result on nasopharyngeal polymerase chain reaction testing for SARS-CoV-2 performed at a local and/or reference laboratory (ie, nucleic acid–based detection). Our primary outcome was occurrence of AKI defined as a 50% increase in Scr concentration over baseline or a 0.3-mg/dL increase from the lowest value within 48 hours, which corresponds to KDIGO (Kidney Disease: Improving Global Outcomes) stage 1 AKI or higher. Baseline Scr level was defined as the lowest Scr measurement within the previous 7 days or the median of all outpatient Scr values obtained within 7-365 days before hospitalization.21 We classified AKI into 3 stages per KDIGO Scr criteria. Patients who required dialysis were classified as having stage 3 AKI. We did not use urine output to define AKI because of the high degree of missingness. We also evaluated secondary outcomes of severe AKI (stage 2/3 AKI including dialysis), death, length of hospital and intensive care unit stay, and AKI recovery. AKI recovery was defined to occur if the last Scr measurement during hospitalization was <1.5 times the baseline value in the absence of dialysis.
Data Sources
We collected information on demographic characteristics, comorbidities (based on International Classification of Diseases codes), procedures, medications, and vital signs. Static features such as sex were extracted at the patient level. Time-varying features such as vital signs and medications were extracted for all time points in which they were measured during the encounter; observations were carried forward through time until a new measurement of the variable was available. We extracted variables from the electronic health record as in prior studies.22 , 23 We validated key features, including dialysis, death, and mechanical ventilation, through manual chart review of a random subsample. Chart review was performed on a targeted random subsample of 20-30 patients flagged as positive for the outcome and the same number of patients who were flagged as negative; these patients’ charts were reviewed manually, and variables were deemed validated if they achieved at least 95% sensitivity/specificity. Data collection ceased at patient discharge or death; therefore, we had complete follow-up on the entire cohort.
Statistical Methods
We present continuous variables as median (interquartile range [IQR]) and categorical variables as count (percentage). We compared categorical variables using χ2 tests and continuous variables using the Wilcoxon rank-sum test between SARS-CoV-2–positive and -negative groups. We calculated absolute risk difference and its 95% CI assuming a binomial distribution.
Our primary analysis was comparison of the incidence of AKI during hospitalization between SARS-CoV-2–positive and -negative patients with unadjusted and adjusted Cox proportional hazards analyses with time-varying covariates, stratified at the hospital level. We defined the start time of observation as hospital admission date and time. Event time was defined as the earliest time point at which AKI criteria were met. We excluded patients with AKI already present at the time of hospital admission because they could not contribute follow-up time. Participants were censored at death, which we assumed to be conditionally independent of AKI, or at end of study (30 d after admission). Patients discharged before 30 days who neither died nor exhibited AKI were assumed to have stable covariates and to be outcome-free after discharge until end of study. We tested the proportionality assumption with manual inspection of log-log survival curves to evaluate whether the curves were parallel. Because the proportionality assumption was not met in the models, hazard ratios (HRs) represent weighted average hazards.24 We present 4 models and report adjusted HRs. Model 1 was a univariable analysis. Model 2 controlled for demographic characteristics (age, sex, race) and comorbidities (congestive heart failure, chronic obstructive pulmonary disease, liver disease, malignancy, hypertension, diabetes mellitus, chronic kidney disease, Elixhauser score25) and the number of days from local pandemic onset (defined as March 1, 2020) to admission date. Model 3 additionally controlled for medication use during hospitalization, including use of angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, aminoglycosides, iodinated radiocontrast agents, diuretic agents, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and vasopressors. Model 4 additionally controlled for vital signs (heart rate, temperature, respiratory rate, oxygen saturation, systolic blood pressure), laboratory results (hemoglobin, white blood cell count, platelet count, glucose, proteinuria, baseline estimated glomerular filtration rate), admission to intensive care unit, and need for mechanical ventilation. The variables describing medication use, vital signs, laboratory results, admission to intensive care unit, and need for mechanical ventilation were updated over time; their value was presumed to be constant until they were replaced by a new measurement. We calculated basic bootstrap 95% CIs for the HRs using 1,000 resampled datasets at the patient level (rather than model confidence intervals) because of violation of noninformative censoring and proportionality assumptions.24 , 26 We performed complete case analysis and did not impute for missing covariates.
We conducted several sensitivity analyses. First, we performed a comparison of the SARS-CoV-2–positive cohort versus a historical cohort of patients hospitalized during the year 2019 to control for changes in patient population characteristics during the pandemic. Second, we included data on covariates and outcomes obtained for patients readmitted within 30 days of initial admission rather than assuming stable covariates and absence of outcome after discharge. Third, we used an alternate definition of AKI using a rolling baseline approach, ie, using only the Scr values obtained during a 48-hour or 7-day rolling window before the current Scr measurement to determine whether AKI develops (using 0.3 mg/dL or 50% increase, respectively, to define AKI). We performed a subgroup analysis to evaluate effects of COVID-19 and AKI for patients who were admitted to the intensive care unit. To test if the association of COVID-19 with AKI changed as the epidemic progressed, we tested the interaction term SARS-CoV-2 positivity and days from first case of COVID-19 to admission on the outcome of AKI. We performed data analysis using SAS (version 9.4; SAS Institute Inc), STATA (release 15; StataCorp LLC), and R (version 4.0.0; R Foundation for Statistical Computing). We defined statistical significance at P < 0.05. We used the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) checklist to ensure high quality of reporting for this cohort study.
Results
Baseline Characteristics
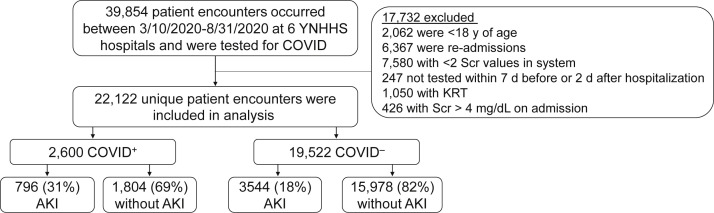
Of the 38,854 patient encounters at 6 Yale New Haven Health system hospitals between March 10, 2020, and August 31, 2020, that included testing for SARS-CoV-2, we included 22,122 in the analysis (Fig 1 ). Of these, 2,600 had tested positive for SARS-CoV-2 and 19,522 had tested negative. Hospitalized patients who tested positive were more likely to be Black (24.8% vs 14.9%) or Hispanic (27.9% vs 12.2%) and more likely to have diabetes (38.3% vs 30.5%), but showed lower prevalences of congestive heart failure and liver disease than patients who tested negative (Table 1 ). The 2 groups had comparable prevalence of chronic kidney disease (16.4% vs 16.6%) and comparable Scr concentration (1 [IQR, 0.8-1.3] vs 1 [IQR, 0.8-1.3] mg/dL), estimated glomerular filtration rate (76.7 [IQR, 52.6-97.1] vs 76.2 [IQR, 55.1-95.9] mL/min/1.73 m2), and serum urea nitrogen level (17 [IQR, 11-26] vs 17 [IQR, 12-25] mg/dL) at admission. Temporal trends in change in SARS-CoV-2 testing and cancellation of elective procedures, SARS-CoV-2 positivity, and medical/surgical service admissions are provided in Figs S1 and S2 and Table S1.
Figure 1.
Flow diagram.
Table 1.
Characteristics at Hospital Admission
| Variable | With COVID-19 (n = 2,600) | Without COVID-19 (n = 19,522) | Standardized Difference |
|---|---|---|---|
| Demographic | |||
| Age | 65.6 [52.5-79.6] | 65.5 [51.7-78] | 0.065 |
| Black race | 646 (24.8%) | 2,916 (14.9%) | 0.25 |
| Female sex | 1,280 (49.2%) | 9,901 (50.7%) | −0.03 |
| Hispanic ethnicity | 725 (27.9%) | 2,375 (12.2%) | 0.401 |
| Comorbidities | |||
| Congestive heart failure | 502 (19.3%) | 4,728 (24.2%) | −0.119 |
| COPD | 831 (32%) | 6,844 (35.1%) | −0.066 |
| Liver disease | 270 (10.4%) | 3,227 (16.5%) | −0.181 |
| Malignancy | 295 (11.3%) | 3,683 (18.9%) | −0.211 |
| Chronic kidney disease | 426 (16.4%) | 3,246 (16.6%) | −0.007 |
| Hypertension | 1,658 (63.8%) | 12,572 (64.4%) | −0.01 |
| Diabetes | 997 (38.3%) | 5,954 (30.5%) | 0.165 |
| Elixhauser comorbidity score | 5 [2-8] | 5 [2-9] | −0.114 |
| Vital signs on admission | |||
| Systolic blood pressure | 132 [118-148] | 137 [121-156] | −0.206 |
| Diastolic blood pressure | 76 [66-85] | 79 [69-89] | −0.196 |
| Pulse rate | 94 [80-109] | 87 [74-102] | 0.314 |
| Respiratory rate | 20 [18-22] | 18 [17-20] | 0.481 |
| Pulse oximetry oxygen saturation | 96 [93-98] | 97 [96-99] | −0.552 |
| Temperature | 98.9 [98-100.4] | 98 [97.5-98.6] | 0.699 |
| Serum laboratory findings on admission | |||
| Serum urea nitrogen, mg/dL | 17 [11-26] | 17 [12-25] | 0.033 |
| Creatinine, mg/dL | 1 [0.8-1.3] | 1 [0.8-1.3] | 0.044 |
| eGFR, mL/min/1.73 m2 | 76.7 [52.6-97.1] | 76.2 [55.1-95.9] | −0.003 |
| Chloride, mEq/L | 100 [97-104] | 102 [99-105] | −0.238 |
| Glucose, g/dL | 123 [105-162] | 120 [102-151] | 0.095 |
| Potassium, mEq/L | 4 [3.7-4.4] | 4 [3.7-4.4] | −0.039 |
| Sodium, mEq/L | 137 [134-140] | 138 [135-140] | −0.082 |
| Hemoglobin, g/dL | 13.2 [11.8-14.4] | 12.8 [11.1-14.2] | 0.212 |
| Platelet count, ×103/μL | 206 [161-262] | 230 [178-289] | −0.212 |
| WBC count, ×103/μL | 6.7 [5.1-9.2] | 9.2 [6.9-12.3] | −0.396 |
| Urinalysis findings | |||
| Specific gravity | 1.02 [1.015-1.027] | 1.018 [1.013-1.026] | −0.013 |
| Proteinuria ≥ 2+ | 598 (33.4%) | 1,790 (15.4%) | 0.429 |
| Blood ≥ 2+ | 354 (19.8%) | 2,184 (19.1%) | 0.019 |
| Leukocytes ≥ 1+ | 486 (27.2%) | 3,504 (30.6%) | −0.075 |
Note: Values for continuous variables given as median [interquartile range]; for categorical variables, as count (percentage). Vital signs, blood laboratory findings, and urinalysis findings are first available during admission.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; WBC, white blood cell.
AKI and Other Adverse Events During Hospitalization
We noted that a higher proportion of patients with COVID-19 experienced AKI compared with those without COVID-19 (30.6% vs 18.2%; absolute risk difference, 12.5% [95% CI, 10.6%-14.3%]; Table 2 ). Patients hospitalized with COVID-19 were more likely to experience stage 2/3 AKI (11.1% vs 4.9%). Among those with AKI, patients with COVID-19 required dialysis more frequently (8.5% vs 3.6%) and for longer durations (10.1 [IQR, 1-21.9] vs 4.1 [IQR, 1-13.7] days). Because the incidence of AKI on hospital admission was not different between those with and without COVID-19 (5.0% vs 4.8%), the difference in AKI was largely driven by hospital-acquired AKI. Fewer patients with COVID-19 had recovered from AKI at the time of discharge from hospital (58% vs 69.8%). Patients admitted with COVID-19 were 5 times as likely to die than others (14.7% vs 3.1%), and these rates were higher in both groups among those in whom AKI developed (29.6% and 11.3%). Patients with COVID-19 had a longer length of stay in the hospital (8 [IQR, 4.5-14.8] vs 4 [IQR, 2.4-7] days).
Table 2.
AKI and Other Outcomes
| Variable | With COVID-19 (n = 2,600) | Without COVID-19 (n = 19,522) | Risk Difference (95% CI) | P |
|---|---|---|---|---|
| AKI | 796 (30.6%) | 3,544 (18.2%) | 12.5% [10.6% to 14.3%] | <0.001 |
| Stage 1 | 508 (19.5%) | 2,579 (13.2%) | 6.3% [4.7% to 7.9%] | |
| Stage 2 | 153 (5.9%) | 594 (3%) | 2.8% [1.9% to 3.8%] | |
| Stage 3 | 135 (5.2%) | 371 (1.9%) | 3.3% [2.4% to 4.2%] | |
| Dialysisa | 68 (8.5%) | 127 (3.6%) | 5% [2.9% to 7%] | <0.001 |
| Time from hospital admission to first dialysis, db | 6.1 [3.7-11] | 5.1 [1.8-12.5] | 1% [−1.1% to 3%] | 0.3 |
| Duration of inpatient dialysis, db | 10.1 [1-21.9] | 4.1 [1-13.7] | 6% [0.9% to 11.1%] | 0.02 |
| AKI on admission | 129 (5.0%) | 941 (4.8%) | 0.2% [−0.9% to 1.0%] | 0.8 |
| ICU admission | 654 (25.2%) | 4,759 (24.4%) | 0.8% [−1% to 2.5%] | 0.4 |
| Length of stay in ICU, dc | 4.9 [1.8-11.1] | 2.3 [1.2-4.7] | 2.6% [1.9% to 3.3%] | <0.001 |
| Ventilator requirementc | 377 (14.5%) | 1,186 (6.1%) | 8.4% [7% to 9.8%] | <0.001 |
| Vasopressor requirementc | 369 (14.2%) | 2,329 (11.9%) | 2.3% [0.8% to 3.7%] | <0.001 |
| AKI recovery (at discharge)a | 462 (58%) | 2,473 (69.8%) | −11.7% [−15.5% to −8%] | <0.001 |
| Length of hospital stay, d | 8 [4.5-14.8] | 4 [2.4-7] | 4% [3.7% to 4.2%] | <0.001 |
| Death | ||||
| Overall | 383 (14.7%) | 612 (3.1%) | 11.6% [10.2% to 13%] | <0.001 |
| Among those with AKIa | 236 (29.6%) | 401 (11.3%) | 18.3% [15% to 21.7%] | <0.001 |
Note: AKI recovery was defined as last serum creatinine measurement before discharge that is <1.5× baseline serum creatinine level.
Abbreviations: AKI, acute kidney injury; COVID-19, coronavirus disease 2019; ICU, intensive care unit.
Includes only those with AKI (n = 4,340).
Includes only those undergoing dialysis (n = 195).
Includes only those admitted to the ICU (n = 5,413).
Factors During Hospitalization Associated With AKI
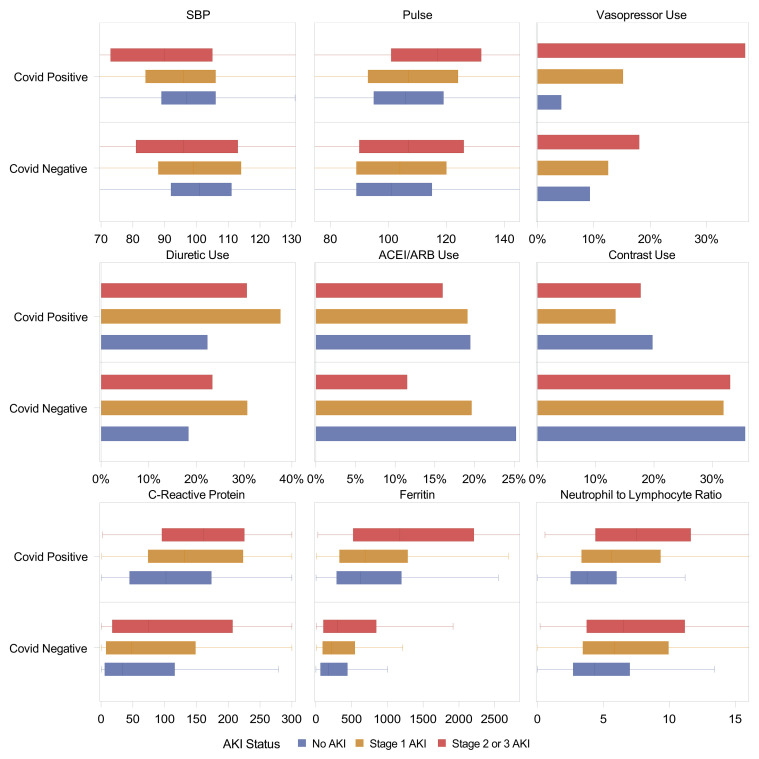
We evaluated various factors associated with AKI in patients with and without COVID-19 (Tables S2 and S3). Patients with COVID-19 had more hypotension (reflected by lower nadir of systolic blood pressure, higher peak heart rate, and greater use of vasopressors), greater diuretic agent use, and higher markers of inflammation such as C-reactive protein and ferritin (Fig 2 ). Radiocontrast agent use was lower in patients with COVID-19 and with AKI. Use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers was lower with greater severity of AKI, but this difference was less pronounced in patients with COVID-19.
Figure 2.
Risk factors of AKI in those with and without COVID-19. Box plots (median, IQR, and whiskers denoting 5th and 95th percentiles) or proportion shown. In patients with COVID-19, all variables are missing < 10% except C-reactive protein (26%). In patients without COVID-19, all variables are missing < 10% except D-dimer (86%), ferritin (86%), and C-reactive protein (85%). All variables are reported as maximum before AKI onset except systolic blood pressure, which is reported as minimum.
Abbreviations: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; AKI, acute kidney injury; COVID-19, coronavirus disease 2019; SBP, systolic blood pressure.
Independent Association of COVID-19 With AKI
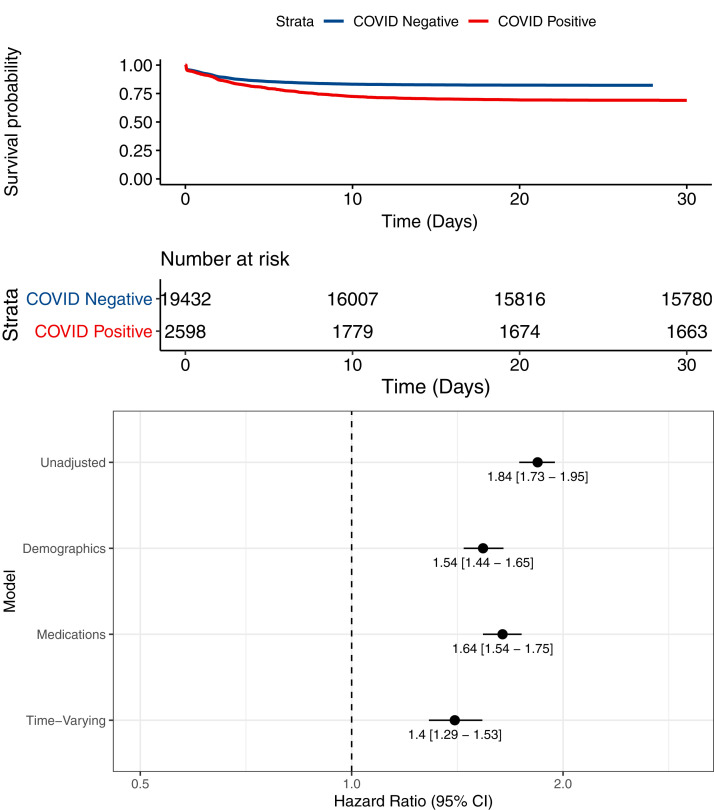
We tested the independent association of COVID-19 with AKI through multivariable adjustment in time-varying Cox proportional hazards models. In unadjusted analysis (stratified by hospital), COVID-19 was associated with an 81% higher rate of AKI (HR, 1.84 [95% CI, 1.73-1.95]; model 1; Fig 3 ). The higher rate persisted after additionally adjusting for demographic characteristics, comorbidities, and time since epidemic onset (adjusted HR, 1.54 [95% CI, 1.44-1.65]; model 2); medication use during hospitalization (adjusted HR, 1.64 [95% CI, 1.54-1.75]; model 3); as well as time-varying factors such as vital signs, laboratory values, intensive care unit admission, and need for mechanical ventilation (adjusted HR, 1.40 [95% CI, 1.29-1.53]; model 4). We did not note a change in association between COVID-19 and AKI over the course of the pandemic (P = 0.4 for interaction).
Figure 3.
Assocation of COVID-19 with AKI. (A) Kaplan-Meier curve for risk of AKI stratified by COVID-19 status. (B) Time-varying Cox proportional hazards models showing association of COVID-19 with AKI. Model 1 is unadjusted. Model 2 includes demographic characteristics (age, sex, race), comorbidities (congestive heart failure, chronic pulmonary disease, livery disease, malignancy, hypertension, diabetes mellitus, chronic kidney disease, and Elixhauser score), and number of days from pandemic onset, defined as March 1, 2020. Model 3 includes model 2 plus medications (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, aminoglycosides, intravenous contrast studies, loop diuretic agents, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and vasopressors). Model 4 includes model 3 plus vital signs (pulse, temperature, respiratory rate, oxygen saturation, systolic blood pressure) and laboratory values (hemoglobin, white blood cell count, platelet count, glucose, urine protein, baseline estimated glomerular filtration rate), intensive care unit status, and invasive ventilation status. All analyses are stratified by hospital.
Sensitivity Analyses
We conducted several sensitivity analyses to test the robustness of our findings. First, we compared rates of AKI in SARS-CoV-2–positive patients versus a group of patients admitted to the hospital 1 year before the onset of COVID-19. Baseline characteristics of patients in the historical cohort were similar to those of patients without COVID-19 in our original cohort (Table S4). AKI and related outcomes in the historical cohort were similar to those in the study patients without COVID-19 (Table S5). COVID-19 was associated with higher rates of AKI with full adjustment in the historical cohort (adjusted HR, 2.06 [95% CI, 1.71-2.39]; Fig S3). Second, we included patient data for patients readmitted within the 30-day censor period (6.6% of those discharged without AKI or death) in whom the assumption of our primary analysis (absence of AKI and stability of covariates after discharge for as long as 30 days) was inaccurate. In this analysis, COVID-19 was associated with higher rates of AKI in the fully adjusted model (adjusted HR, 1.33 [95% CI, 1.21-1.57]; Fig S4). Last, we used an alternate definition for AKI using only Scr values measured during hospitalization as a baseline because we noted differential missingness of outpatient baseline Scr values between the 2 comparison groups (SARS-CoV-2–positive vs -negative, 66% vs 54%). Similar to the primary analysis, COVID-19 was associated with higher rates of AKI in the fully adjusted model (HR, 1.45 [95% CI, 1.32-1.59]; Fig S5).
Subgroup Analysis
One fourth of patients required admission to the intensive care unit in both the SARS-CoV-2–positive and -negative groups (25.2% vs 24.4%). Among these, more patients with COVID-19 required ventilator support (49% vs 24%) and vasopressor support (46% vs 32%), and their length of stay in the intensive care unit was also longer (4.9 [IQR, 1.8-11.1] vs 2.3 [IQR, 1.2-4.7] days; Table S6). Among those admitted to the intensive care unit, patients with COVID-19 had a higher occurrence of AKI (58% vs 32%; absolute risk difference, 25.5% [95% CI, 21.4%-29.5%]). Patients with COVID-19 also had higher incidences of stage 2/3 AKI (39% vs 11%), dialysis-requiring AKI (15% vs 8%), and death (30% vs 10%).
Discussion
In this multicenter, observational cohort study of hospitalized patients, we found that patients with COVID-19 had higher rates of AKI after controlling for multiple risk factors for AKI, including blood pressure, vasopressor and nephrotoxin use, and mechanical ventilation. Patients with COVID-19 also had a higher occurrence of severe AKI and dialysis-requiring AKI, as well as a lower rate of recovery from AKI. Our study adds to the growing evidence that COVID-19 is associated with increased AKI, which may be due to specific direct (eg, infection of kidneys) or indirect (eg, inflammation) effects of COVID-19 on the kidneys.
Our study builds on the increasing evidence associating COVID-19 with AKI. Prior studies showed that AKI occurs in 28%-57% patients hospitalized with COVID-19.1 , 3 , 27 , 28 Similarly, AKI occurred in one third of the patients hospitalized with COVID-19 in our study. Additionally, we found that AKI occurred more frequently in patients with COVID-19 than in those who tested negative for SARS-CoV-2. Patients with COVID-19 also experienced a higher occurrence of more severe forms of AKI, including dialysis-requiring AKI, and fewer patients recovered from AKI. Recently, Fisher et al also showed higher rates of AKI in those with COVID-19 than in those who tested negative for SARS-CoV-2 in New York.3 Although our AKI (and mortality) rates are lower in those with and without COVID-19, the higher rates of AKI in patients with COVID-19 are similar. We believe the lower occurrence of AKI and mortality is likely due to our inclusion of suburban, community, and lower-acuity hospitals, and our findings are similar to another report from a large health system in New York that included such hospitals.1 Finally, our finding that hospitalized patients with COVID-19 had fewer comorbidities than those admitted without COVID-19 was also noted in a prior study.3 In our opinion, this does not represent lower risk of COVID-19 in patients with these comorbidities; instead, this could be explained by 2 factors: patients without comorbidities might decide not to seek medical care during a pandemic and “collider bias,” whereby both COVID-19 and comorbidities (eg, liver disease) lead to hospitalization, resulting in a distorted relationship between comorbidities and COVID-19 when the analysis includes only hospitalized patients.29
We noted several AKI risk factors that were more common in those with COVID-19. Patients with COVID-19 had greater occurrence of hypotension and vasopressor use, greater diuretic agent use, and more severe inflammation. However, use of drugs that are often associated with AKI (eg, radiocontrast agents) and comorbidities were lower in patients with COVID-19. These findings suggest that AKI in COVID-19 may be due to a combination of some typical risk factors for AKI, such as hypotension and volume depletion, as well as some risk factors specific to COVID-19, such as severe inflammation.
Our study has several strengths and adds to existing literature in several important aspects. First, we used a Cox proportional hazards model that accounted for the longer duration of hospitalization in patients with COVID-19. Failure to account for the differential follow-up times could lead to higher detected AKI rates in patients with COVID-19 as a result of higher rates of Scr measurement (ie, ascertainment bias).30 Second, whereas Fisher et al controlled for covariates only on hospital admission, we were able to additionally control for time-updated variables that are expected to change during hospitalization, such as blood pressure, vasopressor use, ventilator use, and medication exposures, which are all risk factors for AKI. Third, our study included a large sample size that allowed us to detect a reasonable difference in AKI rate between cases and controls as well as control for important covariates. Fourth, we verified our findings with several robust sensitivity analyses involving the use of a historical cohort as well as several definitions for AKI and follow-up time. Finally, we included patients who tested positive for SARS-CoV-2 as outpatients and were later admitted to the hospital and thus avoided misclassifying these as SARS-CoV-2–negative.
Our study also had some limitations. First, despite our best efforts to include controls that were similar to cases and control for confounders, important unmeasured confounders may have been missed. We could not control for markers of inflammation as a result of the high degree of missingness in SARS-CoV-2–negative patients. However, it is notable that recent studies showed that the degree of inflammation in patients with COVID-19 was similar to or lower than in those with acute respiratory distress syndrome and sepsis.31 , 32 Second, while our study shows higher rates of AKI in patients with COVID-19, we could not explain the etiology. Third, our selection of a control group may have influenced the association of COVID-19 with AKI. Although we show consistently higher rates of AKI in those with COVID-19 when using historical controls and limiting analysis to patients admitted to the intensive care unit, it is possible that we might have seen a null association if controls were limited to those with a viral respiratory illness caused by agents other than COVID-19. However, our goal was to evaluate if AKI is more prevalent in COVID-19 than in other settings after controlling for known risk factors and mechanisms of AKI (eg, hypotension). Indeed, other viral illnesses (eg, influenza) might be associated with higher incidences of AKI than other settings and might share mechanisms of kidney injury with COVID-19. This will need to be explored in future studies.
Our findings in a large dataset raise several questions for future research. First, the increased risk of AKI needs to be supported by patient-level studies to evaluate biomarkers of kidney injury and inflammation in patients in whom AKI develops with COVID-19. Second, mechanisms of COVID-19–related AKI also need to be evaluated in animal models, and therapeutic agents need to be tested. Finally, we show that fewer patients with COVID-19–related AKI recover from their AKI; whether this will be associated with a higher occurrence of chronic kidney disease needs to be determined because of its impact on patients and health care delivery systems.
COVID-19 in hospitalized patients is associated with a higher rate of AKI after adjustment for a multitude of demographic and clinical variables. Analyses investigating traditional mediators of AKI, such as hypotension and nephrotoxic medications, did not abate this relationship. Further study is warranted of pathophysiologic mechanisms that may mediate kidney injury in COVID-19, as well as the long-term consequences of AKI in COVID-19.
Article Information
Authors’ Full Names and Academic Degrees
Dennis G. Moledina, MD, PhD; Michael Simonov, MD; Yu Yamamoto, MS; Jameel Alausa, Tanima Arora, MBBS; Aditya Biswas, BS; Lloyd G. Cantley, MD; Lama Ghazi, MD, PhD; Jason H. Greenberg, MD, MHS; Monique Hinchcliff, MD, MS; Chenxi Huang, PhD; Sherry G. Mansour, MD, MS; Melissa Martin, MS; Aldo Peixoto, MD; Wade Schulz, MD, PhD; Labeebah Subair; Jeffrey M. Testani, MD, MTR; Ugochukwu Ugwuowo, MBBS, MPH; Patrick Young, PhD; and F. Perry Wilson, MD, MSCE.
Authors’ Contributions
Research idea and study design: DGM, MS, JHG, SGM, MH, FPW; data acquisition: MS, YY, JA, TA, AB, LG, MM, WS, LS, UU, PY; data analysis/interpretation: DGM, MS, YY, AB, LGC, CH, AP, JMT, FPW; statistical analysis: MS, YY, CH; supervision or mentorship: FPW. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
Support
This work was supported by National Institutes of Health grants K23DK117065 (DGM), P30DK079310 (DGM and FPW), and R01DK113191 (FPW). The funders did not play a role in study design, data collection, analysis, reporting, or the decision to submit for publication. Data presented here were obtained through the Yale Department of Medicine’s COVID Explorer data repository funded by the Department of Medicine, the George M. O’Brien Kidney Center at Yale, and resources from the Clinical and Translational Research Accelerator.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received Oct 20, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from a Statistics/Methods Editor and an Associate Editor, who served as Acting Editor-in-Chief. Accepted in revised form December 23, 2020. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
Complete author and article information provided before references.
Figure S1: Proportion of patients with COVID-19 by month.
Figure S2: Medical and surgical admissions by month.
Figure S3: Kaplan-Meier curve and forest plot for sensitivity analysis using historical control data.
Figure S4: Kaplan-Meier curve and forest plot for sensitivity analysis for inclusion of 30-day readmission data.
Figure S5: Kaplan-Meier curve and forest plot for sensitivity analysis using only hospital-acquired AKI.
Table S1: Timeline of SARS-CoV-2 testing and cancellation of elective procedures.
Table S2: Baseline and hospitalization variables by AKI status among patients with COVID-19.
Table S3: Baseline and hospitalization variables by AKI status among patients without COVID-19.
Table S4: Baseline characteristics of historical controls.
Table S5: AKI and other outcomes of patients with COVID-19 compared with historical controls.
Table S6: Subgroup analysis for patients hospitalized in the intensive care unit.
Supplementary Material
Figures S1-S5; Item S1; Tables S1-S6.
References
- 1.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan L., Chaudhary K., Saha A. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher M., Neugarten J., Bellin E. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowe B., Cai M., Xie Y., Gibson A.K., Maddukuri G., Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfarb D.S., Benstein J.A., Zhdanova O. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol. 2020;15(6):880–882. doi: 10.2215/CJN.05180420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy Y.N.V., Walensky R.P., Mendu M.L., Green N., Reddy K.P. Estimating shortages in capacity to deliver continuous kidney replacement therapy during the COVID-19 pandemic in the United States. Am J Kidney Dis. 2020;76(5):696–709.e691. doi: 10.1053/j.ajkd.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng J.H., Hirsch J.S., Hazzan A., Northwell Nephrology COVID-19 Research Consortium Outcomes among patients hospitalized with COVID-19 and acute kidney injury. Am J Kidney Dis. 2021;77(2):204–215.e1. doi: 10.1053/j.ajkd.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan S., Chen L., Yang C.R., Raghuram V., Khundmiri S.J., Knepper M.A. Does SARS-CoV-2 infect the kidney? J Am Soc Nephrol. 2020;31(12):2746–2748. doi: 10.1681/ASN.2020081229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun F., Lutgehetmann M., Pfefferle S. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597–598. doi: 10.1016/S0140-6736(20)31759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jhaveri K.D., Meir L.R., Flores Chang B.S. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98(2):509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Post A., den Deurwaarder E.S.G., Bakker S.J.L. Kidney infarction in patients with COVID-19. Am J Kidney Dis. 2020;76(3):431–435. doi: 10.1053/j.ajkd.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudose S., Batal I., Santoriello D. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golmai P., Larsen C.P., DeVita M.V. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944–1947. doi: 10.1681/ASN.2020050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P., Uppal N.N., Wanchoo R. COVID-19-associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958. doi: 10.1681/ASN.2020050699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutter M., Martin M., Yamamoto Y. Electronic Alerts for Acute Kidney Injury Amelioration (ELAIA-1): a completely electronic, multicentre, randomised controlled trial: design and rationale. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-025117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonov M., Ugwuowo U., Moreira E. A simple real-time model for predicting acute kidney injury in hospitalized patients in the US: a descriptive modeling study. PLoS Med. 2019;16(7) doi: 10.1371/journal.pmed.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siew E.D., Ikizler T.A., Matheny M.E. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moledina D.G., Luciano R.L., Kukova L. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13(11):1633–1640. doi: 10.2215/CJN.04910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moledina D.G., Wilson F.P., Pober J.S. Urine TNF-alpha and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight. 2019;4(10) doi: 10.1172/jci.insight.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stensrud M.J., Hernán M.A. Why test for proportional hazards? JAMA. 2020;323(14):1401–1402. doi: 10.1001/jama.2020.1267. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A., Steiner C., Harris D.R., Coffey R.N. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Burr D. A Comparison of certain bootstrap confidence intervals in the Cox model. J Am Stat Assoc. 1994;89(428):1290–1302. [Google Scholar]
- 27.Pelayo J., Lo K.B., Bhargav R. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10(4):223–231. doi: 10.1159/000509182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed MM, Lukitsch I, Torres-Ortiz AE, et al. Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans. Kidney360. 1(7):614-622. [DOI] [PMC free article] [PubMed]
- 29.Sackett D.L. Bias in analytic research. J Chronic Dis. 1979;32(1-2):51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 30.Lin J., Fernandez H., Shashaty M.G. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10(10):1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remy K.E., Mazer M., Striker D.A. Severe immunosuppression and not a cytokine storm characterize COVID-19 infections. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson J.G., Simpson L.J., Ferreira A.M. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S5; Item S1; Tables S1-S6.