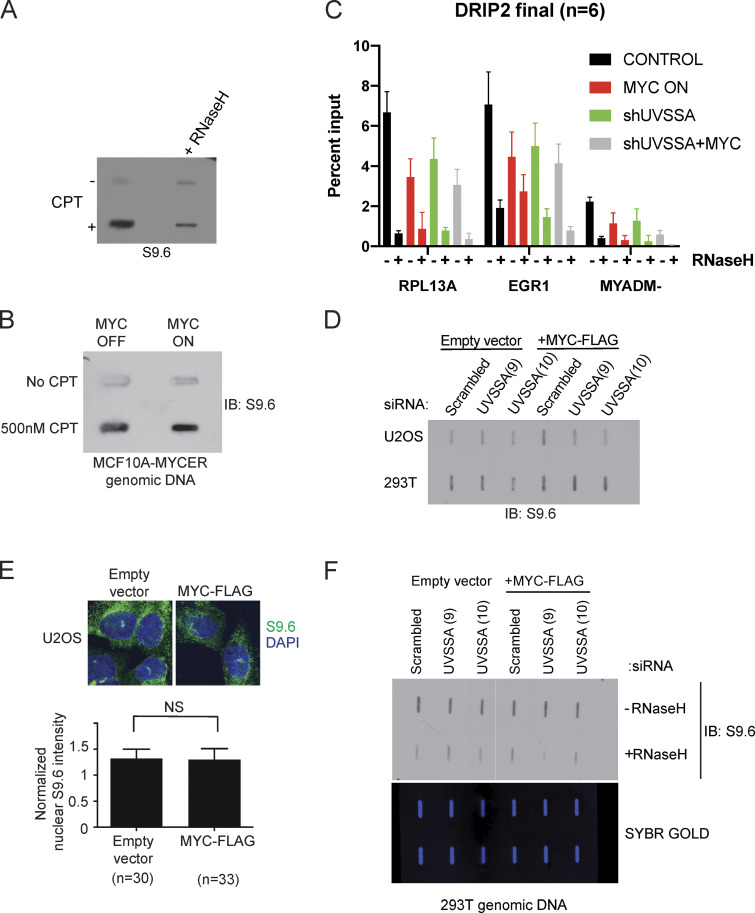
Figure S5.
R-loops do not accumulate upon MYC activation or UVSSA knockdown. (A) Slot blot to examine S9.6 signal specificity. MCF10A-MYC-ER cells were treated with 500 nM camptothecin (CPT) or vehicle for 48 h. Genomic DNA was treated with vehicle or RNase H1 for 1 h, then purified and spotted onto a membrane and incubated with S9.6 antibody. (B) Slot blot to examine S9.6 signal following MYC induction. MCF10A-MYC-ER cells were simultaneously treated with 500 nM CPT or vehicle and 200 nM 4OHT or vehicle to induce MYC-ER and DNA damage for 48 h. Genomic DNA was purified and spotted onto a membrane and incubated with S9.6 antibody. (C) DRIP-qPCR signal upon MYC deregulation and UVSSA down-regulation. RPL13A and EGR1 are known R-loop rich loci, while MYADM- represents a negative control. Error bars represent SEM (n = 3). (D) Slot blot to examine S9.6 levels following MYC induction and UVSSA knockdown in various cell types. U2OS or 293T cells were transfected with siRNA against UVSSA and pcDNA-MYC-FLAG or empty vector for 48 h. Genomic DNA was isolated and subjected to slot blot analysis and staining with S9.6 antibody. (E) Immunofluorescence visualization of R-loops. U2OS cells were transfected with pcDNA-MYC-FLAG and subjected to immunofluorescence using S9.6 antibody after 48 h (top). The indicated number of cells was analyzed for S9.6 intensity in the nuclei (bottom). (F) Slot blot analysis of genomic DNA using S9.6 antibody. Purified genomic DNA harvested from 293T cells transfected with control siRNA or UVSSA siRNA (clones 9 and 10) and pcDNA3-MYC-FLAG or vector was harvested after 72 h. DNA was treated with RNase H1 or vehicle and spotted on a slot blot, and the membrane was incubated with S9.6 antibody (top). Subsequent to imaging, the membrane was stained with SYBR GOLD and visualized (bottom). IB, immunoblot.