Figure 2. Ritonavir‐induced endothelial dysfunction and inflammation are Nox1‐dependent.
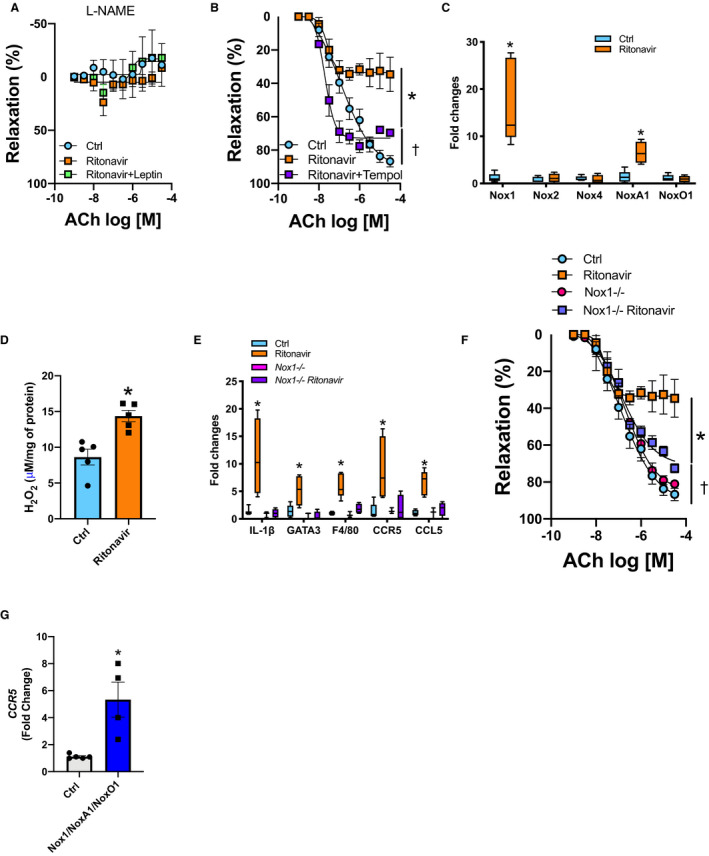
CRC to ACh in aortic rings in the presence of l‐NAME (100 μmol/L) (A) or tempol (100 μmol/L) (B). Real‐time PCR quantification of aortic NADPH oxidases subunits (C). Aortic H2O2 levels measured by Amplex Red (D) from control (Ctrl, vehicle‐treated) and ritonavir‐treated mice (ritonavir, 5 mg/kg per day for 4 weeks, ip) in the presence or absence of leptin treatment (0.3 mg/kg per day for 1 week, via osmotic mini‐pump). Real‐time PCR quantification of inflammatory markers (E) and CRC to ACh (F) in aortic segments from Nox1‐deficient mice (Nox1−/−) treated or not with ritonavir. G, CCR5 gene expression in human umbilical vein endothelial cells transduced with Nox1/NoxA1/NoxO1. Data are presented as mean±SEM. Gene expression data are presented as Min. to Max. N=3 to 8; *P<0.05 vs Ctrl; † P<0.05 vs Ctrl and ritonavir. ACh indicates acetylcholine; Ctrl, control; CCL5, C‐C motif chemokine ligand 5; CCR5, C‐C chemokine receptor 5; CRC, concentration response curves; IL1‐β, interleukin 1‐β; F4/80, the macrophage marker F4/80; GATA3, GATA binding protein 3; IL1‐β, interleukin 1‐β; l‐NAME, l‐NG‐nitro arginine methyl ester; NADPH, reduced nicotinamide adenine dinucleotide phosphate; Nox1, NADPH oxidase 1; and PCR, polymerase chain reaction.
