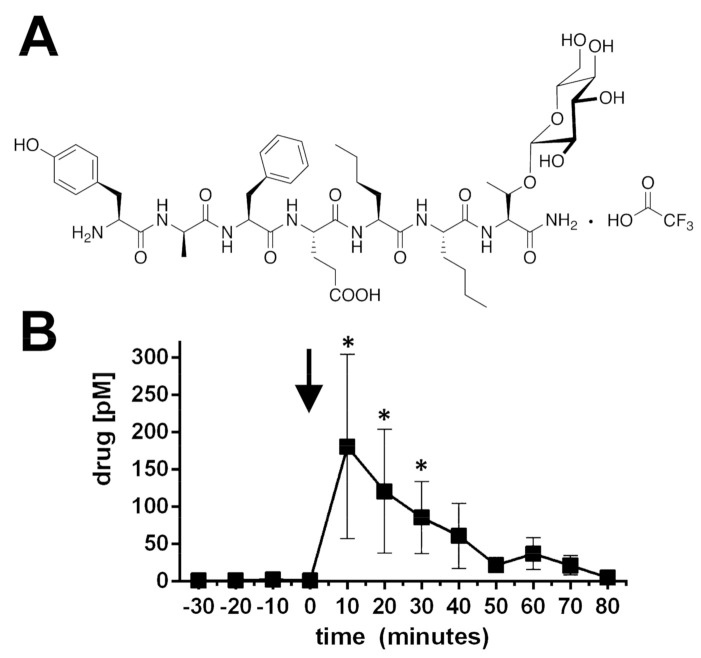
Figure 1.
(A) Structure of BBI-11008. The native deltorphin sequence YaFEVVG-CONH2 was modified to produce the glycopeptide YaFENleNleT(β-D-Glc)-CONH2, where the two L-valine residues have been replaced with L-nor-leucines (Nle), and the terminal glycine has been replaced with the glucoside of L-threonine. Several related glycopeptide structures were screened for MOR and DOR agonism. (B) Proof of blood–brain barrier penetration of the opioid glycopeptide BBI-11008 as determined by microdialysis in the dorsolateral striatum after systemic administration. BBI-11008 (10 mg/kg, i.p.) rapidly reached the dorsolateral striatum as measured by in vivo microdialysis and subsequent mass spectrometric analysis in awake, freely moving rats, implanted 24 h prior to the experiments (fully recovered, wounds healed, covered by a stage that holds the probe in place). Mean concentration ± SEM is plotted against time. A high concentration of BBI-11008 was measured in the dialysate from the first time point after injection (black arrow), with >150 pM at peak level, and remained at active levels at 1 h (~37 pM). For comparison the endogenous opioid peptide leu-enkephalin measured simultaneously was determined to be >20 pM (* p < 0.05, n = 6, repeated measures ANOVA).