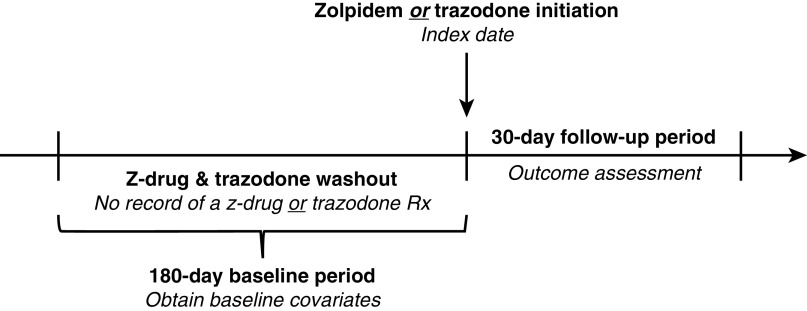
Figure 1.
Study design. Initiators of zolpidem and trazodone were defined as patients who had no record of a z-drug (zolpidem, eszopiclone, zaleplon) or trazodone prescription in the previous 180 days (i.e., the washout period). The index date was defined as the date of study medication initiation. Baseline covariates were identified in the 180-day period before the index date. Study follow-up began immediately after the index date. Rx, prescription.