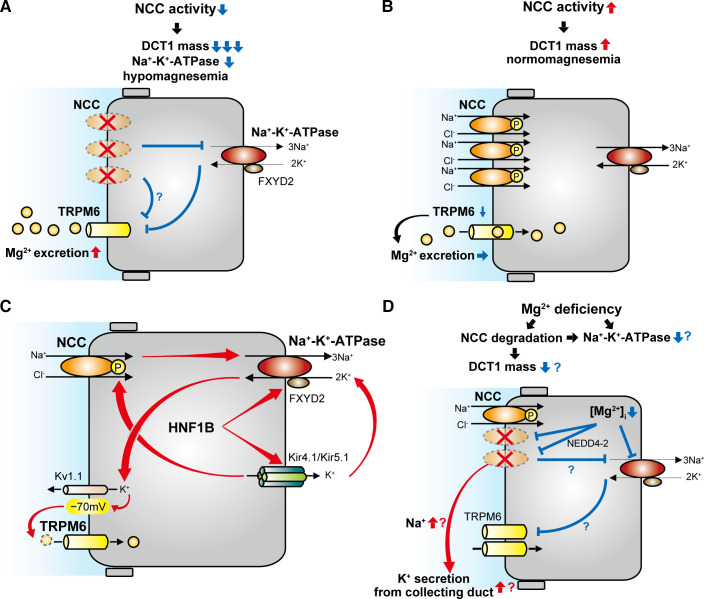
Fig. 2.
Schematic model of the effect of NaCl cotransporter (NCC) activity on distal convoluted tubule (DCT) Mg2+ handling. A: NCC inactivation reduces early DCT (DCT1) mass and Na+-K+-ATPase activity, leading to hypomagnesemia. This is caused by decreased Mg2+ entry through transient receptor potential channel subfamily M member 6 (TRPM6). B: NCC activation increases DCT mass, but TRPM6 per unit DCT1 mass is downregulated, resulting in a minimal effect on Mg2+ entry. C: Na+-K+-ATPase activity is a central determinant of DCT Mg2+ reabsorption because it provides the driving force for Mg2+ entry via TRPM6. Hepatocyte nuclear factor-1β (HNF1B) plays a key role in Mg2+ handling, since it promotes transcription of Na+-K+-ATPase subunit-γ (FXYD2) and Kir5.1, which enhance Na+-K+-ATPase activity. Kir4.1/Kir5.1 activity is closely related to NCC activity. D: Mg2+ deficiency may promote NCC degradation via ubiquitin ligase neuronal precursor cell developmentally downregulated 4-2 (NEDD4-2). Increased Na+ delivery to downstream segments can promote K+ secretion. Mg2+ deficiency upregulates TRPM6 expression, which enhances Mg2+ entry, but NCC downregulation can cause DCT1 atrophy. Combined with reduced levels of the intracellular Mg2+ concentration ([Mg2+]i) for Na+-K+-ATPase activity, this would be expected to reduce Mg2+ entry via TRPM6 channels.