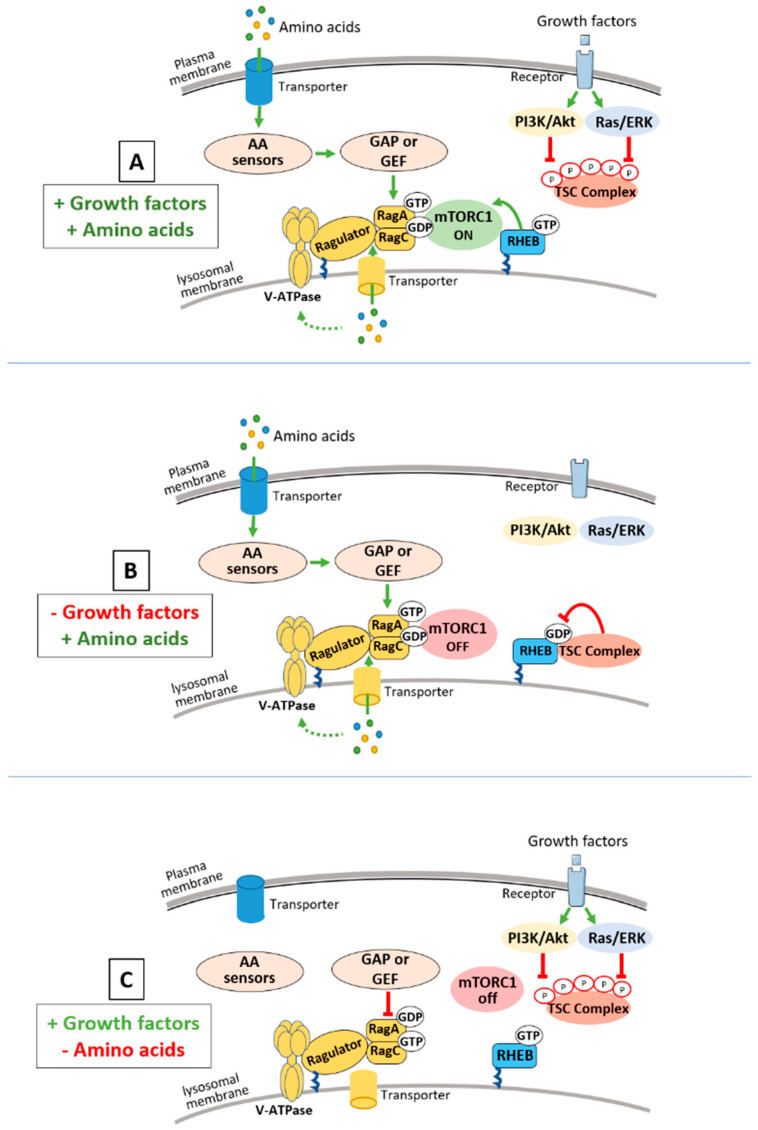
Figure 1.
Mechanistic target of rapamycin complex 1 (mTORC1) integrates signals from growth factors and amino acids. This schematic represents different nucleotide loaded states of the Rheb and Rags GTPases in the presence or absence of amino acid (AA) and Growth factors and their influence on mTORC1 activity. (A) The presence of growth factors and hormones leads to an Akt- or Erk-dependent phosphorylation of tuberous sclerosis complex (TSC), resulting in the immediate release of the TSC complex from the lysosome promoting the accumulation of Rheb-GTP and therefore mTORC1 activation. (B) During a cellular stress or growth factors withdrawal, the TSC complex is recruited to the lysosome where it decreases Rheb-GTP levels and turns off mTORC1 signaling. AAs are sensed via a complex upstream network composed of AA sensors, AA transporters and GAP and GTP exchange factors (GEFs) factors that regulate the nucleotide loaded state of RagA/B and RagC/D. When AAs are present, the Rag heterodimer is converted to its active conformation, RagA/B-GTP and RagC/D-GDP, which leads to the lysosomal recruitment of mTORC1. (Panel C) When AAs are absent, the Rag heterodimer is converted to its inactive conformation RagA/B-GDP and RagC/D-GTP which promotes the release of mTORC1 into the cytoplasm (C). Signals from both AAs and growth factors/hormones are required to promote a full activation of mTORC1.