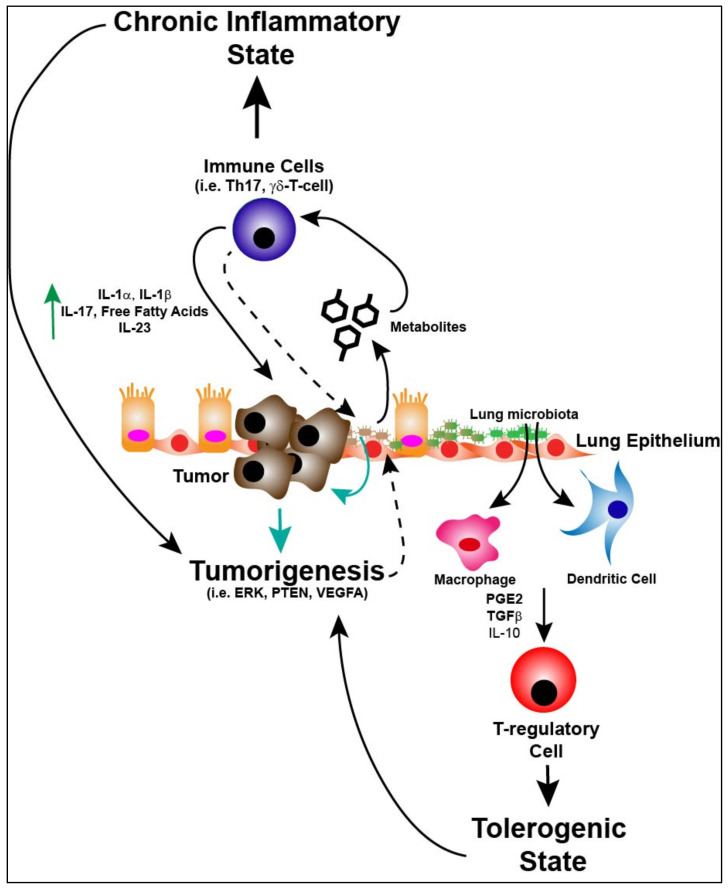
Figure 2.
The lung microbiota as a central mediator of the tumor microenvironment and tumorigenesis. The microbiota of the lung can activate macrophages and dendritic cells in the microenvironment leading to T-reg cell induction through cytokines such as prostaglandin E2 (PGE2), transforming growth factor beta (TGFβ and interleukin-10 (IL-10) which leads to a “tolerogenic” state allowing for not only bacterial propagation and maintenance, but potentially tumor promotion. If respiratory dysbiosis occurs (i.e., from enrichment of upper respiratory tract bacterial species) this can lead to production of metabolic byproducts (i.e., deoxycholic acids, short chain fatty acids, omega 3 polyunsaturated fatty acids, tryptophan) which can induce a pro-inflammatory microenvironment and a “chronic” inflammatory state. Activation of immune cells (i.e., Th17 and γδ-T-cells) leads to production of cytokines (i.e., interleukin-1 alpha (IL-1α), interleukin-1 beta (IL-1β), interleukin-17 (IL-17), free fatty acids and interleukin-23 (IL-23) promoting tumorigenesis. Finally, dysbiosis of the lung microbiota can lead to direct effects on tumor intrinsic factors (i.e., extracellular signal-regulated kinase (ERK), phosphatase and tensin homolog (PTEN), vascular endothelial growth factor A (VEGFA). Additionally, the anatomic and immunologic consequences (dashed lines) of tumor biology change the local microenvironment for lung microbiota, perpetuating respiratory dysbiosis.