Abstract
Background:
Selective serotonin reuptake inhibitors are among the most common agents have been used for the treatment of the premenstrual dysphoric disorder (PMDD); however, due to the diversity in the outcomes and adverse effects, efforts are in progress to find an agent with maximal efficacy and minimal adverse effects. Saffron is an herbal agent consisted of ingredients shown to act as an antidepressant, pain tranquilizer, and antioxidant. In the current study, it is aimed to assess the efficacy of saffron on PMDD treatment.
Materials and Methods:
In the current randomized controlled trial, 120 females with the diagnosis of PMDD were randomly allocated to three groups of treatment with fluoxetine (20 mg, twice daily), saffron (15 mg, twice daily) or placebo for 2 weeks in the luteal phase of two menstruation cycles. Daily record of severity of problems (DRSP) and Hamilton questionnaires had been filled before the interventions and then following the treatment cessation. The questionnaires' scores and drug-related adverse effects were compared among the studied groups.
Results:
Post-intervention assessment of three groups revealed significant improvement in all of the treatment approaches in terms of DRSP and Hamilton assessments (P < 0.001). Although DRSP assessments showed remarkable superiority of saffron to placebo (P = 0.027), Hamilton evaluations showed insignificant differences among the three interventions (P > 0.05). Fluoxetine posed a significantly higher rate of adverse effects as compared to the other agents (P = 0.01).
Conclusion:
Based on the findings of this study, saffron was an efficacious herbal agent for the treatment of PMDD with minimal adverse effects.
Keywords: Crocus, DSM-V, fluoxetine, premenstrual dysphoric disorder
Introduction
Premenstrual dysphoric disorder (PMDD) affects 3%–5% of reproductive age females.[1,2] This cyclic disorder is defined as recurrent mental symptoms, including emotionally lability, depressed mood, aggression, and irritability occurring within the luteal phase of the menstruation cycle.[3] These females are struggling with reduced quality of life due to the negative impacts of PMDD on the social, interpersonal, and occupational aspects of life.[4,5]
The prevalence of PMDD in the community of Iran is compatible to other studies worldwide, as it is estimated that 2.3% of them are resenting from severe symptoms identified as PMDD.[6]
As PMDD has a multifactorial nature, various etiologies have been reported, but it is not fully defined. The cyclic nature of PMDD has deviated the scientists toward the role of a sudden decrease in progesterone level in the luteal phase for this syndrome.[7] Besides, numerous studies have represented that the changes in serotoninergic cascade in the central nervous system are responsible for PMDD symptoms.[8,9] Other theories about the etiology of PMDD include the probable effect of decreased progesterone on neurotransmitters such as gamma-aminobutyric acid or serotonin, the influences of increased prolactin, or oversensitization to the prolactin, abnormality in the hypothalamic-pituitary-adrenal axis or alteration in glucose metabolism such as insulin resistance.[10,11,12]
Selective serotonin reuptake inhibitors (SSRIs) are the most common regimens used for PMDD treatment shown to affect varieties of PMDD-related complications, including physical symptoms, psychosocial performance, work preferences and quality of life, positively.[13,14,15]
There are studies in the literature representing the role of herbal therapies on the relief of the PMDD-related symptoms. Even an American study has shown that up to 80% of American females prefer using herbal agents merely or in combination with other chemical agents for PMDD.[16] Crocus sativus L, popularly known as saffron, has been shown to efficaciously improve mild-to-moderate symptoms of depression.[17,18,19] In general, it is suggested that the main components of Saffron, crocin, and safranal can potentially inhibit the reuptake of dopamine, norepinephrine, and serotonin; a fact that ignited a theory about the use of saffron for the management of PMDD.(1)
Although the role of saffron for the treatment of depression has been well-established, there are few studies assessing saffron efficacy for the treatment of PMDD. In the current study, we have aimed to compare saffron with fluoxetine as the most popular SSRI used for PMDD, for the management of this disorder.
Materials and Methods
Study population
This is a randomized clinical trial conducted on 120 reproductive age females with the definite diagnosis of PMDD based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) in Alzahra and Khorshid Hospitals affiliated at Isfahan University of Medical Sciences from April 2017 to February 2018.
The diagnosis of PMDD based on DSM 5 in reproductive-aged females (20–45 years old) with normal body mass index of 19.8–25, regular menstrual cycles of 21–35 days, and menstrual period of 3–10 days was considered as inclusion criteria.
Unmet criteria were considered as the recent use of antidepressants, contraceptives and vitamin supplements, pregnancy or lactation, or the incidence of misfortune events such as divorce or grief.
Patients who were not adherent to the study protocol, and who represented her reluctance for participation/continue the study were excluded from the study.
Isfahan University of Medical Sciences Ethics Committee approved the study protocol. Besides, it was approved by the Iranian Registry of Clinical Trials code number IRCT20180602039946N1. The study was entirely explained for the study population, and they were reassured about the confidentiality of their personal information; eventually, the written consent was obtained.
Interventions
The study population was recruited through convenience sampling. Then they were randomly divided into three groups of treatment with fluoxetine, saffron, and placebo using Random Allocation software; therefore, each of the cases was provided with a number allocated her to one of the groups.
The remedies were given to the patients in blinded capsules and by a nurse who was aware of the content of the capsules; therefore, the patients and the psychiatrist who interviewed and filled the questionnaires were blinded to the intervention.
The first group was treated with fluoxetine, 20 mg twice daily (Abidi Company, Tehran, Iran), the second group with saffron, 15 mg twice daily (Novin Saffron, Mashad, Iran),[20] and the third group with placebo made of starch similar in shape, color, and size with two other capsule made by Pharmacy Faculty of Isfahan University of Medical Sciences. In order to control the odor, all of the capsules were fragranced by saffron odor. The participants used the agents for 2 weeks in the luteal phase of the menstrual cycle. In this term, the treatments were administered during the luteal phase within 14 days before the expected day of the next menses and until the 1st day of active bleeding. These treatment approaches performed for two menstrual cycles. All cases were recommended to initiate the treatments when their symptoms get started.
Primary outcomes
Age, marital status, educational level, medical history, and occupation were recorded in the study checklist for all of the study participants.
Hamilton and daily record of severity of problems (DRSP) were the means of assessing the participant's severity of symptoms and then assessing the efficacy of treatments. The questionnaires had been filled before the interventions and then following the cessation of the interventions.
The adverse effects following the use of each agent, including gastrointestinal symptoms, headache, anxiety, insomnia, and increased menorrhagia, were recorded in the checklist as well.
Means of evaluation
Hamilton questionnaire is a means of assessing depression symptoms. It contains 21 questions with scorings of 0 as no symptom to 4 as severe symptoms. The score of 0–7 is defined as regular, 8–13 as mild, 14–18 as moderate, 19–22 as severe, and >23 as very severe depression.[21,22]
Endicott et al. in 1996 presented the DRSP questionnaire. This questionnaire has the Cronbach's alpha of 0.95 and divides the symptoms into five subtypes of anxiety, depression, emotional, retention, and physical. The scoring system of the symptoms includes; no symptoms: Zero; notifying symptoms without chores interruption: (1) the presence of symptoms partially causing an interruption in the daily activity but not job absence: (2) and complete interruption in daily activities: (3) The severity of the symptoms was scored from 7 days before the menstruation to at most 4 days following the bleeding. The scores of 0–33 were defined as mild, 33–66 as moderate, and >66 as severe.[23,24]
A psychiatrist responsible for the study that was blinded to the therapeutic approach of each patient interviewed the participants and filled the questionnaires.
Statistical analysis
The obtained data were entered into the Statistical Package for Social Sciences (version 22, IBM Corporation, Armonk, NY, USA). The descriptive data were presented in mean and standard deviation, absolute numbers, and percentages. For inferential data, ANOVA, Tukey test, t-test, ANCOVA test, and Chi-square test were used. The P < 0.05 was considered as statistically significant level.
Results
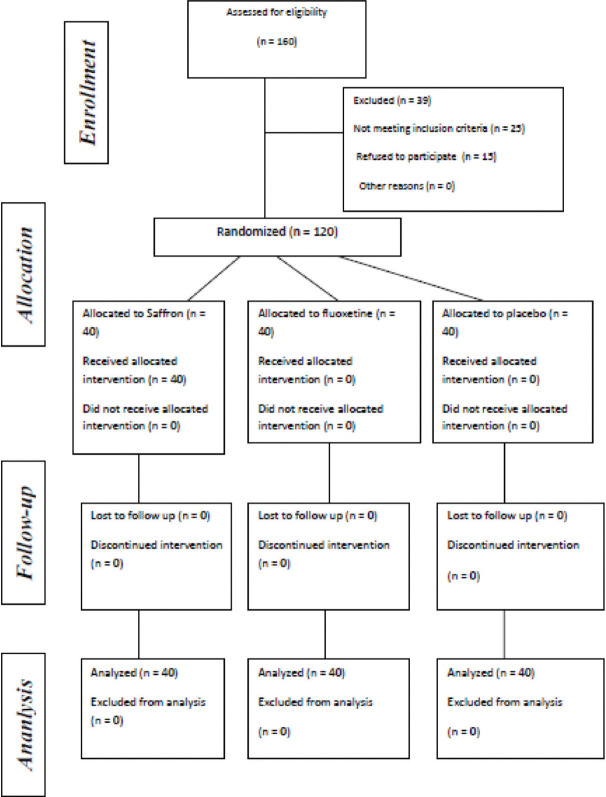
In the current study, 120 females were randomly divided into three groups of treatment with fluoxetine, saffron, and placebo in three equal groups consisted of 40 members [Figure 1].
Figure 1.
Consort diagram of the study
The three assessed groups were similar in terms of demographics including age (P = 0.48), marital status (P = 0.49), educational level (P = 0.32), and occupation (P = 0.65). Detailed information is demonstrated in Table 1.
Table 1.
Comparison of demographic information in the three assessed groups
| Variables | Saffron group (%) | Fluoxetine group (%) | Placebo group (%) | P | Test |
|---|---|---|---|---|---|
| Age | 31.65±4.69 | 32.72±5.14 | 31.45±5.27 | 0.48 | ANOVA |
| Marital status | |||||
| Single | 15 (37.5) | 20 (50.0) | 19 (47.5) | 0.49 | χ2 |
| Married | 25 (62.5) | 20 (50.0) | 21 (52.5) | ||
| Educational level | |||||
| Primary school | 0 (0.0) | 1 (2.5) | 1 (2.5) | 0.32 | Fisher’s exact test |
| Under diploma | 7 (17.5) | 7 (17.5) | 7 (17.5) | ||
| Diploma | 11 (27.5) | 13 (32.5) | 11 (27.5) | ||
| Bachelor of science | 12 (30.0) | 14 (35.0) | 19 (47.5) | ||
| Master of science | 10 (25.0) | 5 (12.5) | 2 (5.0) | ||
| Occupation | |||||
| Homemaker | 14 (35.0) | 16 (40.0) | 18 (45.0) | 0.65 | Fisher’s exact test |
| Employee | 26 (65.0) | 23 (57.5) | 22 (55.0) | ||
| Self-employed | 0 (0.0) | 1 (2.5) | 0 (0.0) |
The assessment of DRSP revealed statistically significant differences among the groups before the interventions (P = 0.011) as the scores of the fluoxetine-treated group were remarkably higher than placebo-treated patients (P = 0.008). The covariance analysis for post-intervention measurements showed significantly higher scores of placebo-treated cases than saffron-treated ones (P = 0.027). All of the three groups represented a significant decrease in their DRSP scores following the interventions as compared to the scores before the intervention initiation (P < 0.001). Hamilton assessments revealed insignificant differences among the groups both before and following the interventions (P > 0.05), while all of the three groups showed remarkably improved scores following the interventions (P = 0.001) [Table 2].
Table 2.
Comparison of daily record of severity of problems and Hamilton scores before the interventions and after the intervention among the studied population
| Variables | Saffron group | Fluoxetine group | Placebo group | P | P (between saffron group versus group fluoxetine) | P (between saffron group versus placebo group) | P (between fluoxetine group versus group placebo) |
|---|---|---|---|---|---|---|---|
| DRSP | |||||||
| Before intervention | 52.76±10.30 | 56.43±10.90 | 49.60±8.76 | 0.011 | 0.23 | 0.33 | 0.008 |
| After intervention | 29.66±16.52 | 35.05±15.21 | 33.46±15.50 | 0.022 | 0.99 | 0.02 | 0.08 |
| Change | −22.59±11.40 | −21.45±12.20 | −15.30±11.70 | 0.022 | 0.91 | 0.027 | 0.076 |
| P | <0.001 | <0.001 | <0.001 | ||||
| Hamilton | |||||||
| Before intervention | 10.50±2.02 | 10.78±1.47 | 10.60±1.41 | 0.75 | 0.74 | 0.96 | 0.88 |
| After intervention | 5.46±2.97 | 6.72±2.98 | 6.80±2.81 | 0.095 | 0.18 | 0.13 | 0.99 |
| Change | −4.97±2.46 | −4.02±2.24 | −3.71±2.62 | 0.080 | 0.23 | 0.08 | 0.85 |
| P | <0.001 | <0.001 | <0.001 |
DRSP: Daily record of severity of problems
Table 3 demonstrates the DRSP and Hamilton scores regarding the classification of the tests into mild, moderate, and severe. Based on this table, the severity of DRSP scores was significantly different among the three groups before the interventions (P = 0.006). The fluoxetine-treated group prominently presented a higher rate of severe symptoms before the interventions. The status of Hamilton scores severity was not statistically different among the groups before the interventions (P = 0.77), and also after the interventions (P = 0.16).
Table 3.
Comparison of the three interventions regarding the severity of daily record of severity of problems and Hamilton scores
| The severity of the symptoms | Saffron group (%) | Fluoxetine group (%) | Placebo group (%) | P | Test |
|---|---|---|---|---|---|
| DRSP before the interventions | |||||
| Mild (0-33) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.006 | χ2 |
| Moderate (33-66) | 33 (82.5) | 27 (67.5) | 38 (95.0) | ||
| Severe (>66) | 7 (17.5) | 13 (32.5) | 2 (5.0) | ||
| DRSP after the interventions | |||||
| Mild (0-33) | 25 (67.6) | 15 (41.7) | 19 (54.3) | 0.18 | Fisher’s exact test |
| Moderate (33-66) | 11 (29.7) | 20 (55.6) | 15 (42.9) | ||
| Severe (>66) | 1 (2.7) | 1 (2.8) | 1 (2.9) | ||
| Hamilton score before intervention | |||||
| Regular (0-7) | 2 (5.0) | 1 (2.5) | 0 (0.0) | 0.77 | Fisher’s exact test |
| Severe (8-14) | 38 (95.0) | 39 (97.5) | 40 (100.0) | ||
| Hamilton score after interventions | |||||
| Regular (0-7) | 29 (78.4) | 21 (58.3) | 22 (62.9) | 0.16 | χ2 |
| Severe (8-14) | 8 (21.6) | 15 (41.7) | 13 (37.1) |
DRSP: Daily record of severity of problems
A comparison of the three groups in terms of the presence/absence of intervention-related adverse effects revealed a significantly higher rate of side effect occurrence in the fluoxetine group (P = 0.01). Besides, the detailed comparison of the side effects showed a remarkable difference between the groups (P = 0.01), as well [Table 4].
Table 4.
Comparison of drug-related adverse effects in the study population
| Variables | Saffron group, n (%) | Fluoxetine group, n (%) | Placebo group, n (%) | P | Test |
|---|---|---|---|---|---|
| Adverse effects (no/yes) | 32 (80)/8 (20) | 19 (47.5)/21 (52.5) | 25 (62.5)/15 (37.5) | 0.01 | χ2 |
| Type of adverse effects | |||||
| None | 32 (80.0) | 19 (47.5) | 25 (62.5.5) | 0.01 | Fisher’s exact test |
| Gastrointestinal symptoms | 2 (5.0) | 8 (20.0) | 9 (22.5) | ||
| Headache | 0 (0.0) | 3 (7.5) | 3 (7.5) | ||
| Anxiety | 0 (0.0) | 4 (10.0) | 1 (2.5) | ||
| Insomnia | 1 (2.5) | 3 (7.5) | 0 (0.0) | ||
| Increased menorrhagia | 5 (12.5) | 3 (7.5) | 2 (5.0) |
Discussion
PMDD is known as the severe course of PMS; based on DSM-V is defined as the existence of one of the five affective symptoms, anxiety, irritability, marked anger, affective lability, and depressed mood, plus five or more symptoms related to mentioned affective manifestations.[25] Varieties of therapeutic approaches have been proposed for the treatment of PMDD, while due to the response rate or side effects of the therapeutic agents, the scientists are probing for the best approach. To the best of our knowledge, the present study is the first one assessing the use of saffron for the treatment of PMDD. In the current report, the studied groups were similar in terms of age, marital status, educational level, and occupation; therefore, the demographic information may affect the intervention outcomes were similar among the studied population. Our findings represented the superiority of saffron to placebo in terms of DRSP, but Hamilton assessments. Besides, a comparison of saffron with fluoxetine, as one of the SSRIs routinely used for PMDD treatment,[26] revealed an insignificant difference. Further evaluations showed a significantly higher rate of adverse effects in the fluoxetine-treated females than the other two groups.
Beiranvand et al. conducted a case–control study on females resenting from PMS. They prescribed 30 mg of extracted saffron once daily for two menstrual cycles versus placebo, and eventually found significant improvement in the severity of PMS symptoms for both of the groups, while the comparison of the cases and controls revealed the significant superiority of saffron to placebo.[27]
An earlier report by Agha-Hosseini et al. in 2008, represented considerable symptoms improvement following the treatment either with placebo or saffron prescribed in 30 mg daily doses divided into two 15 mg capsules. The comparison of placebo with saffron showed remarkable superiority of saffron in both DRSP and Hamilton scoring systems.[20]
Serotoninergic system dysregulation in the luteal phase has been estimated to play the most significant role in the PMS presentations. In addition, there are studies in the literature representing the effect of sex hormones, progesterone in particular, on the uptake, transport, binding, and turnover of serotonin. Therefore, most of the attention has been deviated toward the role of serotonin for the cyclic symptoms known as PMS or for severe cases as PMDD.[28,29] In this regard, there are studies in the literature showing the efficacy of saffron for the treatment of mild-to-moderate depression;[17,19] even the study of Akhoundzadeh et al. showed insignificant difference between imipramine as one of the most common agents for the treatment of depression versus saffron considering response to the treatment.[18] The safranal and crocin are the two ingredients extracted from saffron shown to play the antidepressant effects of this herbal agent through serotoninergic system pathways.[30]
The pain relief by the saffron has been well-established in the controlling of labor pain and renal colic, as well.[31] Besides, it has been shown that saffron can be as effective tranquilizer, antispasmodic, and anxiolytic agent as benzodiazepines.[32,33] Flavonoids and carotenoids are the other ingredients derived from saffron with antioxidant effect through the trapping of free radicals and prevention from prostaglandin production; therefore, in addition to the antioxidant nature, other features of saffron use may be attributed to these ingredients.[34,35] Summing mentioned features up together shows the values of saffron for the treatment of PMDD.
In summary, by consideration of two aspects, symptom relief, and adverse effects, we found saffron to be as effective as fluoxetine, and superior to placebo. Nevertheless, the small size of the studied population and the short duration of the study are the limitations of our study for the generalization of the outcomes. Further studies are strongly recommended.
Limitations
There may be some confounding lifestyle-related variables that are neglected in assessing the efficacy of each regimen in the management of PMDD; therefore, further detailed information about the number of deliveries, age of puberty and type of contraception may lead to more valuable results. Another limitation of our study is failure to follow the patients to assess the agents' duration of effectiveness. Therefore, further studies are recommended.
Conclusion
Based on the findings of this study, saffron was an efficacious herbal agent for the treatment of PMDD with minimal adverse effects.
Financial support and sponsorship
The study was sponsored by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We are grateful to Alzahra and Khorshid Hospitals' outpatient clinic employees. We also acknowledge Dr. Ali Safaei for his efforts in the writing and technical preparation of the current paper.
References
- 1.Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002;32:119–32. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]
- 2.de Carvalho AB, Cardoso TA, Mondin TC, da Silva RA, Souza LD, Magalhães PV, et al. Prevalence and factors associated with premenstrual dysphoric disorder: A community sample of young adult women. Psychiatry Res. 2018;268:42–5. doi: 10.1016/j.psychres.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien PM, Bäckström T, Brown C, Dennerstein L, Endicott J, Epperson CN, et al. Towards a consensus on diagnostic criteria, measurement and trial design of the premenstrual disorders: The ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13–21. doi: 10.1007/s00737-010-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennerstein L, Lehert P, Bäckström TC, Heinemann K. The effect of premenstrual symptoms on activities of daily life. Fertil Steril. 2010;94:1059–64. doi: 10.1016/j.fertnstert.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hamzawi SA, Mohammed BK. Prevalence, socio-demographic variables, symptoms profile, and comorbidity of premenstrual dysphoric disorder. Int J Res Pharm Sci. 2019;10:694–8. [Google Scholar]
- 6.Delara M, Borzuei H, Montazeri A. Premenstrual disorders: Prevalence and associated factors in a sample of Iranian adolescents. Iran Red Crescent Med J. 2013;15:695–700. doi: 10.5812/ircmj.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraemer GR, Kraemer RR. Premenstrual syndrome: Diagnosis and treatment experiences. J Womens Health. 1998;7:893–907. doi: 10.1089/jwh.1998.7.893. [DOI] [PubMed] [Google Scholar]
- 8.Yonkers KA, Simoni MK. Premenstrual disorders. Am J Obstet Gynecol. 2018;218:68–74. doi: 10.1016/j.ajog.2017.05.045. [DOI] [PubMed] [Google Scholar]
- 9.Rapkin A. A review of treatment of premenstrual syndrome and premenstrual dysphoric disorder. Psychoneuroendocrinology. 2003;28(Suppl 3):39. doi: 10.1016/s0306-4530(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 10.Firoozi R, Kafi M, Salehi I, Shirmohammadi M. The relationship between severity of premenstrual syndrome and psychiatric symptoms. Iran J Psychiatry. 2012;7:36–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Mishell DR., Jr Premenstrual disorders: Epidemiology and disease burden. Am J Manag Care. 2005;11:S473–9. [PubMed] [Google Scholar]
- 12.Taavoni S, Barkhordari F, Goushegir A, Haghani H. Effect of Royal Jelly on premenstrual syndrome among Iranian medical sciences students: A randomized, triple-blind, placebo-controlled study. Complement Ther Med. 2014;22:601–6. doi: 10.1016/j.ctim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: Epidemiology and treatment. Curr Psychiatry Rep. 2015;17:87. doi: 10.1007/s11920-015-0628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sepede G, Sarchione F, Matarazzo I, Di Giannantonio M, Salerno RM. Premenstrual dysphoric disorder without comorbid psychiatric conditions: A systematic review of therapeutic options. Clin Neuropharmacol. 2016;39:241–61. doi: 10.1097/WNF.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 15.Pearlstein T. Treatment of premenstrual dysphoric disorder: Therapeutic challenges. Expert Rev Clin Pharmacol. 2016;9:493–6. doi: 10.1586/17512433.2016.1142371. [DOI] [PubMed] [Google Scholar]
- 16.Wyatt KM, Dimmock PW, Frischer M, Jones PW, O'Brien SP. Prescribing patterns in premenstrual syndrome. BMC Womens Health. 2002;2:4. doi: 10.1186/1472-6874-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L.in the treatment of mild to moderate depression: A double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–51. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 18.Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi AH, Khalighi-Cigaroudi F. Comparison of Crocus sativus L.and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial [ISRCTN45683816] BMC Complement Altern Med. 2004;4:12. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, Jamshidi AH. Hydro-alcoholic extract of Crocus sativus L.versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized pilot trial. J Ethnopharmacol. 2005;97:281–4. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Agha-Hosseini M, Kashani L, Aleyaseen A, Ghoreishi A, Rahmanpour H, Zarrinara AR, et al. Crocus sativus L.(saffron) in the treatment of premenstrual syndrome: A double-blind, randomised and placebo-controlled trial. BJOG. 2008;115:515–9. doi: 10.1111/j.1471-0528.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Association AP. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Amazoncom. The United States of Americ American Psychiatric Pub. 2013 [Google Scholar]
- 23.Endicott J, Nee J, Harrison W. Daily record of severity of problems (DRSP): Reliability and validity. Arch Womens Ment Health. 2006;9:41–9. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- 24.Kialashaki A, Shokouhi F, Tofighi M, Zafari M, Zarenegad N. Effect of lavandula essence on premenstrual syndrome. J Mazandaran Univ Med Sci. 2012;22:48–56. [Google Scholar]
- 25.Ozcan H, Oral E, Gulec M, Turkez H, Gulec TC, Ustundag MF, et al. Total oxidant–antioxidant and paraoxonase-1 levels in premenstrual dysphoric disorder: A follow-up study. Psychiatry Clin Psychopharmacol. 2017;27:116–24. [Google Scholar]
- 26.Maharaj S, Trevino K. A comprehensive review of treatment options for premenstrual syndrome and premenstrual dysphoric disorder. J Psychiatr Pract. 2015;21:334–50. doi: 10.1097/PRA.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 27.Beiranvand SP, Beiranvand NS, Moghadam ZB, Birjandi M, Azhari S, Rezaei E, et al. The effect of Crocus sativus (saffron) on the severity of premenstrual syndrome. Eur J Integr Med. 2016;8:55–61. [Google Scholar]
- 28.Shoupe D. Diagnosis and Treatment of Premenstrual Syndrome: Differentiating PMS from Premenstrual Dysphoric Disorder PMDD and Premenstrual Exacerbation Disorder PMED.Handbook of Gynecology. University of Southern California, Los Angeles, CA, USA. 2017:181–5. [Google Scholar]
- 29.Mattina GF, Steiner M. Premenstrual Dysphoric Disorder.Women's Mental Health. Springer. 2020:73–93. [Google Scholar]
- 30.Kleinstäuber M, Witthöft M, Hiller W. Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: A meta-analysis. J Clin Psychol Med Settings. 2012;19:308–19. doi: 10.1007/s10880-012-9299-y. [DOI] [PubMed] [Google Scholar]
- 31.Azhari S, Ahmadi S, Rakhshandeh H, Jafarzadeh H, Mazlom SR. Evaluation of the effect of oral saffron capsules on pain intensity during the active phase of labor. Iran J Obstet Gynecol Infertil. 2014;17:1–10. [Google Scholar]
- 32.Marder M, Estiú G, Blanch LB, Viola H, Wasowski C, Medina JH, et al. Molecular modeling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABAA receptor complex. Bioorganic Med Chem. 2001;9:323–35. doi: 10.1016/s0968-0896(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 33.Ai J, Dekermendjian K, Wang X, Nielsen M, Witt MR. 6-Methylflavone, a benzodiazepine receptor ligand with antagonistic properties on rat brain and human recombinant GABAA receptors in vitro. Drug Dev Res. 1997;41:99–106. [Google Scholar]
- 34.Boskabady MA, Aslani M. Relaxant effect of Crocus sativus (saffron) on guinea-pig tracheal chains and its possible mechanisms. J Pharm Pharmacol. 2006;58:1385–90. doi: 10.1211/jpp.58.10.0012. [DOI] [PubMed] [Google Scholar]
- 35.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–4. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]