Abstract
Natural Antisense Transcripts (NATs) are long non-coding RNAs (lncRNAs) that overlap coding genes in the opposite strand. NATs roles have been related to gene regulation through different mechanisms, including post-transcriptional RNA processing. With the aim to identify NATs with potential regulatory function during fly development, we generated RNA-Seq data in Drosophila developing tissues and found bsAS, one of the most highly expressed lncRNAs in the fly wing. bsAS is antisense to bs/DSRF, a gene involved in wing development and neural processes. bsAS plays a crucial role in the tissue specific regulation of the expression of the bs/DSRF isoforms. This regulation is essential for the correct determination of cell fate during Drosophila development, as bsAS knockouts show highly aberrant phenotypes. Regulation of bs isoform usage by bsAS is mediated by specific physical interactions between the promoters of these two genes, which suggests a regulatory mechanism involving the collision of RNA polymerases transcribing in opposite directions. Evolutionary analysis suggests that bsAS NAT emerged simultaneously to the long-short isoform structure of bs, preceding the emergence of wings in insects.
Author summary
Long non-coding RNAs (lncRNAs) are transcribed regions of the genome that, unlike coding genes, are not translated to proteins. Mostly undetected until the recent development of advanced sequencing methods to profile the RNA content (the transcriptome) of the cells, we know now that in many species they rival in number protein coding genes. In the fly genome, there are more than two thousand lncRNAs, most of which of unknown function. Here we characterize the function of bsAS, one of the most abundant lncRNAs encoded in this genome. We show that bsAS is essential for the correct development of the fly wings, since these become very aberrant and ill-formed in mutants in which we delete this gene. We also show that the function of bsAS in the development of fly wings is mediated by the regulation of the expression of the blistered gene, a protein coding gene that overlaps in the fly genome with bsAS.
Introduction
In recent years, the annotation of long non-coding RNAs (lncRNAs) has expanded substantially thanks to the spread of high-throughput RNA sequencing technologies [1]. Natural Antisense Transcripts (NATs) are most commonly defined as fully processed lncRNAs which overlap protein coding genes on the opposite strand with or without exonic complementarity [1]. Several roles in genomic regulation (in cis or in trans) have been reported for NATs in metazoans [2,3], including gene expression regulation of the host protein coding gene, DNA methylation, chromatin modifications and RNA editing. NATs have been related to different human diseases, such as cancer, parkinsonism, alzheimer or autism and they have become even more relevant since they emerged as putative targets for gene therapy (revised in [4]). NATs have also been related to regulation of alternative splicing in vertebrates [5]. In a well understood case, a transcript antisense to Zeb2 promotes intron retention of the sense gene and affects its translation in human epithelial-mesenchymal transition [6].
The fruit fly genome is estimated to encode more than two thousand lncRNAs [7], of which about one third are NATs. However, only few have a characterized function. For instance, roX is a well-studied antisense transcript involved in chromatin regulation and required for dosage compensation of the male X chromosome [8]. Or the male specific abdominal (msa), a lncRNA expressed specifically in fly male abdomen, has been related to fertility in flies [9]. Here, we investigate the role of NATs in the regulation of alternative splicing during Drosophila melanogaster development. We have specifically identified a lncRNA (CR44811), the expression of which regulates the isoform usage of the sense transcription factor blistered/Drosophila Serum Response Factor (bs/DSRF, or simply bs gene), a gene essential for the proper development of wings. To form the final wing during fly development, the wing disc undergoes a process of evagination and ultimately develops a characteristic patterning of veins and interveins (reviewed in [10]). Veins are epithelial formations that carry the trachea and nerves of the adult wing [11] and their specification is tightly regulated during the fly development. Vein and intervein regions are specified from the third instar larvae through the action of bs/DSRF, which has been reported to be expressed specifically in intervein regions [10]. Blistered mutants show dramatic defects in the wings, such as reduction of the intervein tissue, and overgrowth and appearance of extra veins [12–14]. A part from its role in wing morphogenesis, bs has also been related to neural processes such as sleep, visual orientation and working memory in Drosophila [15,16]. In mammals, the homologue SRF gene has been involved in muscle development [17] and neuronal plasticity [18], among other processes.
The protein encoded by bs, DSRF, is, in fact, present in two different isoforms in the fly, which differ in length. We found that the long isoform of bs is preferentially expressed in the eye, while the short isoform is preferentially expressed in the wing, particularly in intervein regions. We show that the usage of these isoforms is regulated in a tissue-specific manner by the expression of the bs NAT CR44811 (herein bsAS). The transcription of bsAS, which occurs specifically in intervein regions, impairs the expression of the long isoform of bs, promoting, in consequence, the relative expression of the short isoform. The overexpression of the long isoform in bsAS mutants induces the formation of extra vein tissue in adult wings. Our results also suggest that the differential regulation of bs expression in different tissues could be driven by the usage of tissue-specific promoters. In particular, the bs promoter specifically active in wing intervein regions appears to interact, through the formation of a genomic loop, with the bsAS promoter. Phylogenetic analysis shows that the emergence of bsAS within arthropoda is contemporary to the functional distinction between short and long bs isoforms and precedes the appearance of the wings in insects.
Material and methods
Drosophila strains
Fly strains used for this work are: wild-type (CantonS), nub-GAL4; UAS-GFP and salE/Pv-GAL4 (kindly gifted by Corominas’ lab, UB), w1118 (kindly gifted by Gebauer’s lab, CRG), bs-GAL4 (B67082), rn-GAL4/TM3 (B7405), UAS-RNAi_bs_long (B26755) and bsAS -/-, UAS-bsAS, UAS-bsIsoB and UAS-bsLongIsos (generated in the lab). Flies were grown in standard media at 25°C.
In situ hybridization and immunohistochemistry
In situ hybridizations and immunostaining were carried out according to standard protocols. For in situ hybridization, bsAS DNA probe was synthesized by conventional PCR using a PCR DIG Labeling Mix (Roche). A biotinylated sense primer was used to bind the PCR product to streptavidin beads. Anti-sense probe was purified by denaturalization of the DNA attached to the beads. Primers used for probe synthesis are listed in S4 Table. Peroxidase conjugated anti-digoxigenin and Tyramide signal amplification (TSA, Life Technologies) were used for fluorescent in situ hybridization (FISH). DSRF immunostaining was performed with mouse anti-DSRF (Active Motif, 1:200). Fluorescently labeled secondary antibody anti-mouse Alexa 594 was from Life Technologies. Discs were mounted in SlowFade (Life Technologies) supplemented with 1 μM DAPI (Life Technologies) to label nuclei. Third instar larvae wing and eye discs were analyzed with a SPE confocal microscope (Leica) at the Advanced Light Microscopy Unit of the Centre for Genomic Regulation (CRG, Barcelona, Spain). For all FISH and immunostainings from 6 to 12 imaginal discs were analyzed. Quantification of DSRF staining from vein and intervein regions was performed with ImageJ software. Intervein region between veins 3 and 4, and vein 3 were selected for quantification and average fluorescence intensity was obtained. For discs overexpressing bs long isoform under the control of rotund driver, a comparable region was selected.
Chromatin immunoprecipitation
As starting material, 100 wt and bsAS-/- third instar larvae wing discs were used per experiment. Imaginal discs were manually dissected and pooled in 1 mL PBS-0.01% TritonX100. Formaldehyde was added to a final concentration of 1% and tissues were fixed for 10 minutes at room temperature in a rotating wheel. Sonication was performed in a Diagenode Bioruptor for 15 minutes at high intensity with ON/OFF alternate pulses of 30 seconds. Sheared chromatin was aliquoted and flash frozen in liquid nitrogen. Chromatin immunoprecipitation assays were next performed as previously described [19]. For immunoprecipitation, 2 μg of anti-H3K4me3 (Abcam/ab8580) were used. Enrichments were analyzed by Real-Time PCR. Primers used for ChIP enrichment detection are listed in S4 Table. Three independent biological replicates were performed per experiment.
RNA isolation, library preparation and sequencing
RNA from 20 to 40 imaginal discs was extracted with ZR-RNA MicroPrep Kit from Zymo Research following the manufacturer’s instructions. Sequencing libraries were prepared using TruSeq Stranded mRNA Library Preparation Kit from Illumina and following the manufacturer’s instructions. Sequencing was performed in a HiSeq sequencer from Illumina at the Ultrasequencing Unit of the Centre for Genomic Regulation (CRG, Barcelona, Spain). A minimum of 50 million paired-end 75 bp-long reads were obtained per replicate and two replicates were performed per each tissue.
Cell isolation from wing imaginal discs
From 150 to 200 third instar larvae wing imaginal discs from animals expressing the GFP under the bs driver were dissected and disaggregated in 10× trypsin solution (Sigma, T4174) for 1 hour at room temperature, as previously described [20]. To discard dead cells, DAPI was added to the samples at a 1 μg/ml final concentration. Cells were sorted according to the GFP intensity on an Influx sorter (BD), in the Flow Cytometry Unit, from the Centre for Genomic Regulation (CRG) and University Pompeu Fabra (UPF, Barcelona, Spain). GFP positive and negative cells were isolated and RNA was extracted as above.
Retro-transcription and Real-Time PCR analyses
Retro-transcriptions and qPCRs were performed as described previously [19]. Primers used for Real-Time PCR are listed in S4 Table. From two to five independent biological replicates were performed per experiment.
CRISPR-Cas9-induced deletion in flies
Guide RNAs around the bsAS TSS were designed using the CRISPR Target Finder from the flyCRISPR portal (http://flycrispr.molbio.wisc.edu/). Sequences of the gRNAs are listed in S4 Table. gRNAs were cloned into a BbsI digested pU6-BbsI-chiRNA vector [20] following the protocol in flyCRISPR portal. A mixture of pU6-BbsI-gRNA1 and pU6-BbsI-gRNA2 was injected into embryos expressing Cas9 protein under the control of the vasa driver. Injection was performed in Rainbow Transgenic Flies Inc. (Camarillo, USA). Injected flies (F0) were crossed in groups of 4 males and females. F1 flies were allowed to mate and ley eggs for 10 days before screening. Sequential crosses were performed until the flies presenting the deletion were identified and isolated. Mutant flies were crossed with flies carrying a CyO balancer and homozygous mutants were isolated (S2A Fig). Flies were screened by conventional PCR. Briefly, genomic DNA was extracted from groups of 4 flies by smashing the animals in lysis buffer containing 0.5% NP40, 10 mM TrisHCl pH8.0, NaCl 150 mM, EDTA 2 mM and proteinase K 1 mg/mL. Genomic DNAs were incubated for 2 h at 50°C and centrifuged at top speed for 5 minutes to remove the remaining fly fragments. 2 μL of the extracts were used for each PCR. Primers used for the screening are listed in S4 Table.
Transgenic constructs
For transgenesis, bs isoform B and long isoform (common to isoforms A and C) cDNAs were amplified by rtPCR (primers used for the amplifications are listed in S4 Table) and inserted by Gibson cloning into a pUAST-AttB vector digested with EcoRI. Transgenes were integrated using the phi31 integrase system in fly strain J36, in the chromosome III. Injection for transgenesis was performed in the Drosophila Injection Service from the Institut de Recerca Biomèdica (IRB, Barcelona, Spain).
Chromosome Conformation Capture (3C)
Chromosome conformation capture protocol was adapted from previous reports [21]. For each experiment a 3C library and a genomic library were performed. For genomic libraries, genomic DNA from 5 third instar larvae was extracted by smashing the animals in lysis buffer containing 0.5% NP40, 10 mM TrisHCl pH8.0, NaCL 150 mM, EDTA 2 mM and proteinase K 1 mg/mL. Genomic DNA was incubated for 2 h at 50°C, centrifuged at top speed for 5 minutes to remove the remaining larval fragments, purified by performing two rounds of phenol:chloroform extraction and precipitated with ethanol. For 3C libraries, 150 wing imaginal discs were manually dissected and pooled in 1 mL PBS-0.01% TritonX100. Formaldehyde was added to a final concentration of 1% and tissues were fixed for 10 minutes at room temperature in a rotating wheel. Discs were washed in 1 mL of PBS-0.01% TritonX100-0.125 M glycine for 5 minutes at room temperature and finally resuspended in 500 μL of lysis buffer (10 mM Tris pH 8, 10 mM NaCl, 0.2% NP40 1x Complete Protease Inhibitor Cocktail) and flash frozen in liquid nitrogen. To perform the libraries, 5 μg of genomic DNA and the fixed 150 wing discs were equilibrated in 164 μL of digestion mix (20 μL HhaI 10x buffer, 5 μL 10% SDS and 139 μL H2O) for 1 h at 37° in a ThermoMixer. 32 μL of 10% TritonX100 were added to each library and incubated for 1 h at 37°C. Digestion was performed by adding 2 μL of HhaI restriction enzyme (New England Biolabs) and incubating for 2 h at 37°C. After first digestion, 2 μL of restriction enzyme were added and samples were incubated over night at 37°C. Digestions were inactivated for 20 minutes at 65°C and cooled on ice. To perform the ligation, 165 μL of ligation mix (2x T4 ligation buffer + 4 μL T4 ligase) were added to the libraries. Ligations were incubated for 4 h at 18°C. Libraries were de-crosslinked by adding 2 μL of proteinase K 20 mg/mL and incubated over night at 65°C. Remaining RNAs in the samples were removed by RNase treatment for 30 minutes at 37°C. Libraries were finally purified by phenol:chloroform extraction and precipitated in ethanol. Quantification of interactions was performed by Real-Time PCR, comparing the interaction observed in the regions of interest to a known interacting region (positive control). Primers used for Real-Time PCRs are listed in S4 Table. At least, three independent biological replicates were performed per experiment.
RNA-Seq processing
Data was processed using grape-nf (available at https://github.com/guigolab/grape-nf). RNA-Seq reads were aligned to the fly genome assembly dm6 [22] using STAR 2.4.0 software [23] with up to 4 mismatches per paired alignment using the FlyBase genome annotation r6.05 [7]. Only alignments for reads mapping to ten or fewer loci were reported. Gene and transcripts TPMs were quantified using RSEM [24]. Tracks were visualized as a Track Hub at the UCSC Genome Browser [25].
NATs in the fly genome and in larval tissues
Antisense lncRNAs were inferred from their overlap and orientation relative to protein coding genes. A minimum overlap of 1 exonic nucleotide was required to classify the pairs into the following configurations: (i) 5' head-to-head: first exon overlap; (ii) 3' tail-to-tail: last exon overlap; (iii) internal: lncRNA is within the protein coding gene; (iv) external: protein coding gene is within the lncRNA; and (iv) complex: overlap of more than 2 genes.
Pairs of ncRNAs antisense to mRNAs with more than 1 isoform and expressed at least 0.5 TPMs in at least both replicates of any L3 sample were selected for further analyses. Ratio of exon coverage over the gene coverage was computed using bwtool summary [26]. Candidate pairs were ranked based on the Pearson's coefficient of correlation of exon ratio and antisense expression and on the standard deviation of exon ratios. Exons showing a standard deviation higher than 0.1 and an absolute correlation higher than 0.7 were selected.
Differential gene expression analysis of mutant versus wild type samples
Pairwise differential gene expression (DEG) analysis between wild type and mutant samples was performed using EdgeR [27]. Only genes expressed at least 5 TPM in at least two samples were selected for this analysis. DEG selected showed log2 fold change > 2 and FDR < 0.01. Biological Processes from Gene Ontology database enriched in upregulated and downregulated datasets were assessed by using GOstats [28] and visualized with ReviGO [29], with a p value cutoff of 0.001.
Promoter and ChIP-Seq analysis
To search for transcription factor binding sites (TFBSs) in bs and bsAS TSSs we used the Promo 3.0 tool [30]. We selected TFBSs with a matrix dissimilarity rate less or equal than 5% that were present both in bs TSS1 and bsAS TSS but not in bs TSS2. Available ChIP-Seq data of GAF [31], RNA PolII [32], Pc and Ph [33] were aligned to the fly genome (dm6) using GEM-Mapper [34] with up to 2 mismatches per read using the dm6 genome assembly [7,22]. ChIP-Seq data from modENCODE TFs were visualized at UCSC Genome Browser from the ENCODE Portal [35].
Evolutionary conservation
To track down the evolutionary conservation of bs and bsAS, we first analyzed RNA-Seq data of whole body and head of female Drosophila species from modENCODE (GSE44612 [36]). Next we investigated the expression of bs and bsAS in stranded RNA-Seq data of Apis mellifera (whole body: GSE83437 [37] and head: GSE87001 [38]; UCSC apiMel2 assembly; modelRefGene genome annotation available at UCSC), in stranded RNA-Seq data of Anoplophora glabripennis (PRJNA274806;) in unstranded RNA-Seq data of Folsomia candida (PRJNA239929; RefSeq assembly accession: GCF_002217175.1; NCBI Folsomia candida Annotation Release 100) and in stranded RNA-Seq of Daphnia pulex (GSE103939; assembly GCA_000187875.1; annotation ENSEMBL release-26). Finally, we manually annotated bs isoforms across metazoans taking advantage of available RNA-Seq data and relying on split reads to properly annotate the exon junctions, which were visualized with ggsashimi [39]: Anopheles gambiae (GSE55453 [40]; GSE59773 [41]), Apis mellifera (GSE52289 [42]; GSE65659 [43]; SRP068487), Daphnia pulex (DRP002580), Strigamia maritima (SRP041623), Tetranychus urticae (GSE31527 [44,45]), Crassostrea gigas (GSE31012 [46]; SRP058882), Gallus gallus (GSE41338 [47]), and Nematostella vectensis (GSE46488 [48]). Homo sapiens bs annotation was directly extracted from GENCODE v19 [49]. Data was processed using grape-nf (available at https://github.com/guigolab/grape-nf) with the same parameters as our RNA-Seq data.
Results
Natural antisense transcription in Drosophila melanogaster
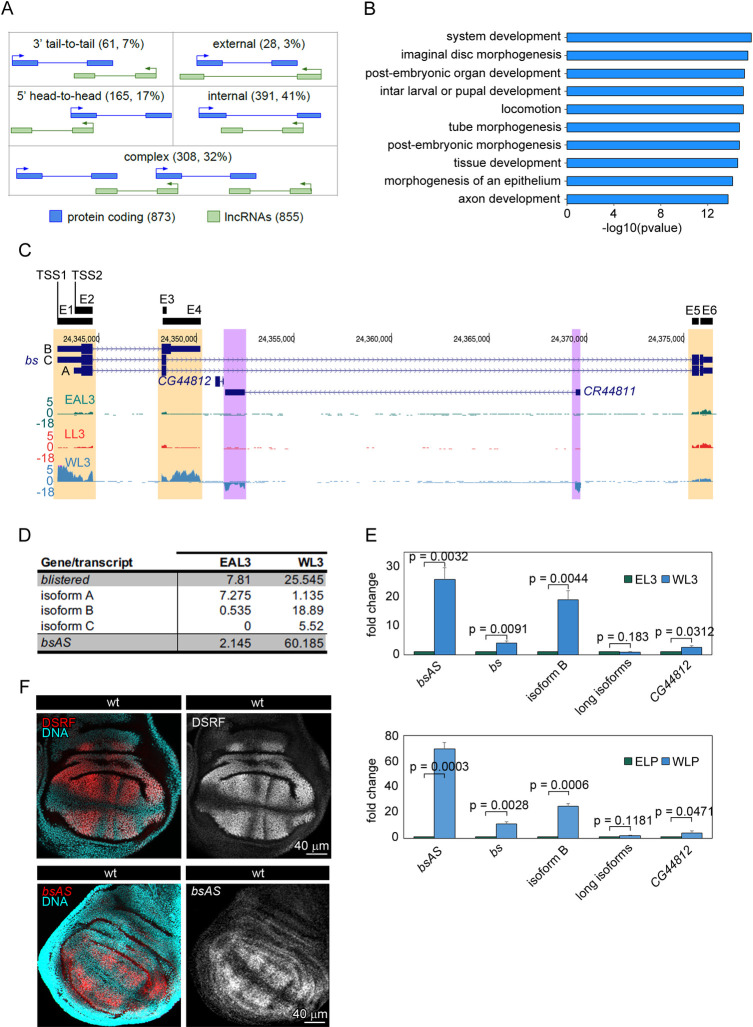
The D. melanogaster genome encodes 16,698 genes, including 13,920 protein coding genes, 2,433 lncRNAs and 308 pseudogenes (FlyBase v6.05 [7]). Although antisense lncRNAs are not explicitly annotated, they can be inferred from their overlap and orientation relative to protein coding genes. We calculated that 855 lncRNAs (35%) overlap 873 protein coding genes in antisense orientation (Natural Antisense Transcripts, NATs), forming 953 sense/antisense (SA) pairs, a number in line with previous reports [50,51]. The coding (sense) and non-coding (antisense) gene pairs, can be arranged in different configurations (Fig 1A): in most of the pairs, the lncRNA is fully included within the protein coding gene (391 pairs, 41%), followed by 5' head-to-head pairs (165 pairs, 17%) and 3' tail-to-tail pairs (61 pairs, 7%). Only in a minority of the pairs (28 pairs, 3%), the protein coding gene is fully included in the lncRNA, while the remaining 32% of the pairs (308) are arranged in complex configurations involving three or more genes (Fig 1A). Remarkably, fly protein coding genes involved in SA pairs are significantly associated to developmental and morphogenetic processes (Fig 1B). This strongly suggests that NATs might play a relevant regulatory role in fly development.
Fig 1. Natural antisense transcription in Drosophila melanogaster.
(A) Configuration of sense/antisense (SA) pairs identified in D. melanogaster genome. We have identified 855 lncRNAs overlapping 873 coding genes in the fruit fly annotation. Around a 32% of the SA pairs present a complex configuration, meaning that one lncRNAs overlaps more than one coding gene or the other way around. (B) Gene Ontology Term Enrichment analysis of coding genes overlapping antisense transcripts. Sense genes are mainly related to development and morphogenesis categories. (C) Expression of the bs locus in the different tissues. Isoform B of bs is expressed mainly in wings, correlating with the highest expression of the antisense lncRNA CR44811. In the other tissues, the isoform A seems to be the predominant one. In the leg, also some expression of the lncRNA is observed. Regions showing differences in expression between tissues are highlighted (bs in orange and CR44811 in purple). (D) Expression of bs isoforms and bsAS in eye-antenna and wing imaginal discs at third instar larvae. The expression values, expressed in TPMs, represent the mean of two independent biological replicates. (E) Relative expression of bs and bsAS in wings and eyes at third instar larvae (L3 –upper panel–) and late pupa (LP –lower panel–) stages. bsAS and the short isoform B of the coding gene are much higher expressed in wings than in eyes, whereas there are not significant differences in the expression of the long isoforms. The protein coding gene CG44812, of unknown function, is almost not expressed in these tissues. Error bars depict Standard Error of the Mean (SEM) of at least three biological replicates. Statistical significance was computed by one-sample t-test. (F) Expression pattern of DSRF and bsAS in wt wing imaginal discs. Upper panels, immunostaining of DSRF (red and grey) in wt WL3. DSRF is expressed in the intervein region of the wing pouch. Lower panels, in situ hybridization of bsAS (red and grey) in WL3. The expression of bsAS mimics DSRF expression pattern in the intervein regions. Nuclei, stained with DAPI, are depicted in blue in all cases.
With the aim of identifying NATs with potential regulatory function during fly development, we performed strand-specific RNA-Seq of three imaginal tissues of D. melanogaster third instar larvae (L3): eye-antenna (EAL3), leg (LL3) and wing (WL3) (S1A Fig and S1 Table). Of the 953 SA pairs, we found 145 (about 15%) in which the two members of the pair were detected with more than 0.5 TPMs (Transcripts per Million Mapped Reads [24]) in at least one tissue, albeit not necessarily the same. Since splicing regulatory activity has been documented for a human NAT [6] and extensive relationship between antisense transcription and splicing has been hypothesized in human [5], we explored specifically the relationship between NAT expression and alternative transcript usage across fly larval samples. Of the 145 SA pairs with both genes expressed in at least one tissue in L3, 104 pairs (72%, 102 genes) involve a protein coding gene with multiple isoforms. We compared the expression of these NATs with the inclusion of the 964 exons of the sense protein coding genes, focusing on the exons the inclusion of which changed the most (see Material and Methods). We found a few cases of relationship between expression of the NAT and the splicing of the sense protein coding genes—the most notable being the case of the bs/DSRF gene (S1B Fig).
The bs/DSRF gene encodes for two protein isoforms, both carrying the DNA binding domain MADS-box, but differing at the terminal end, one much longer than the other (449 vs. 355 amino acids). The long isoform is encoded by two transcripts (variants A and C in Fig 1C), that use two different transcription start sites (TSS2 and TSS1, respectively), and differ only in the first exon. The other protein isoform is encoded by a shorter two-exon transcript (variant B), which shares TSS1 with isoform C. The two long isoforms contain a 26 Kb intron, which holds the NAT CR44811 (bsAS from now onwards due to its overlap with the bs gene), and a coding gene of unknown function (CG44812).
The RNA-Seq data reveals, a contrasting pattern of bs isoform expression between wing and eye and leg tissues (Fig 1C and 1D and S2 Table). First, the long isoforms of bs are globally expressed at similar levels in eye, leg and wing imaginal discs. However, in the eye, only isoform A is expressed, while wing mostly expresses isoform C (Fig 1D). Second, the short isoform B is only expressed in the wing, and at much higher levels than the long isoforms. Therefore, bs is globally much more expressed in the wing than in the eye and the leg. Third, the NAT bsAS also shows contrasting expression between these tissues, being very highly expressed in wing—where it is one of the most highly expressed lncRNAs in the fly genome (S1C Fig) —but very low in eye and leg imaginal discs. We independently confirmed the expression of the bs isoforms and of bsAS by qPCR in wing and eye at third instar larvae and late pupa stages (Fig 1E). The restricted expression of bsAS in wing imaginal discs is also supported by the presence of trimethylation of lysine 4 of histone H3 (H3K4me3, mark associated to active promoters) in wing, but not in eye imaginal discs (S1D and S1E Fig). In situ hybridization in wing imaginal discs reveals that the expression of bsAS is restricted to the intervein regions, like that of bs (Fig 1F), suggesting that these two genes may be co-regulated in wing imaginal discs. Since isoforms B and C share TSS1, and isoform A is associated to TSS2, these observations also suggest that TSS1 is mostly active in the wing, and TSS2 is mostly active in the eye.
bsAS controls bs isoform usage during fly development
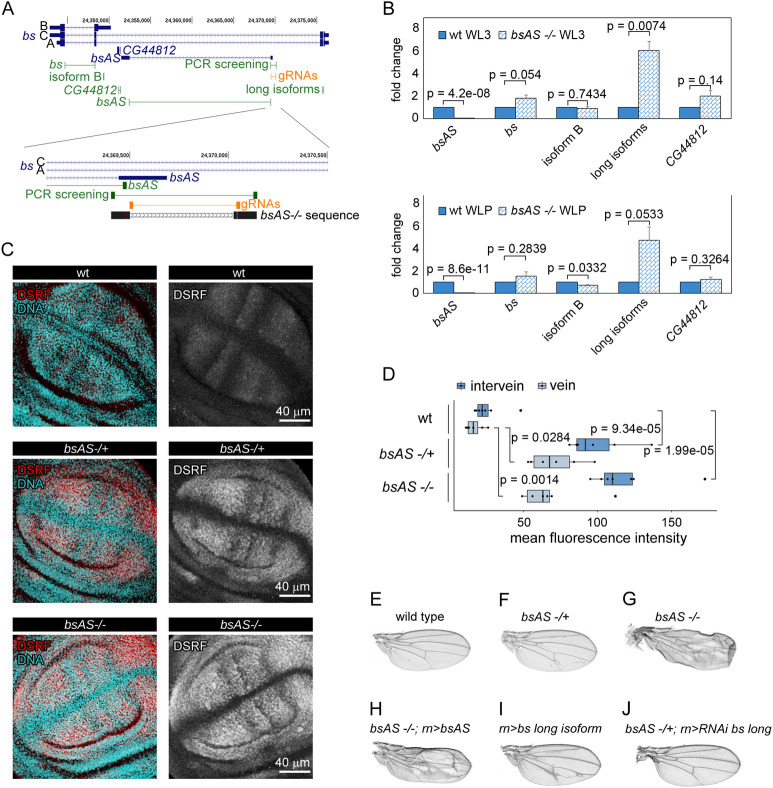
In summary, thus, in wing, bsAS is highly expressed, coinciding with the high expression of the short isoform B. In contrast, in eye, bsAS is not expressed, nor short isoform B. Since the expression of bsAS seems to be associated to the expression of short isoform B, this suggests that bsAS could play a role in regulating isoform usage of bs. To test this hypothesis, we used CRISPR/Cas9 to induce a 560 bp deletion surrounding the TSS of bsAS (Figs 2A and S2). This, effectively abolishes expression of this gene in L3 and LP in wing imaginal discs (Fig 2B). Knocking out bsAS has little effect on the expression of the short isoform B, but induces overexpression of the long isoforms. As a result, there is an overall increase in the expression of bs in the bsAS mutant wings compared to wt (Fig 2B). This increase is particularly significant at protein level, both in heterozygous and homozygous bsAS mutant wings, and it is observed both at intervein and vein regions (Fig 2C and 2D). Although the expression of bsAS in the eye imaginal disc is very low, its deletion induces the overexpression of DSRF in few determined cells posterior to the morphogenetic furrow, but this does not produce any observable phenotype in adult animals (S3A and S3B Fig). Animals carrying the deletion exhibited, in contrast, notable defects in wing development, which are much stronger in homozygous mutant flies, and resembling those of the bs mutant flies [12–14] (Fig 2E, 2F and 2G). These were blistered, presenting necrotic regions and strong defects in vein/intervein patterning, in particular, extra vein tissue in intervein regions.
Fig 2. bsAS controls bs isoform usage during fly development.
(A) Distribution of primers and CRISPR gRNAs along the bs locus. Upper panel, primers amplifying the different isoforms of bs and bsAS are depicted in green; gRNAs used to induce the deletion of bsAS are depicted in orange. Lower panel, zoom of the lncRNA TSS region. Sanger sequence of bsAS mutant flies is depicted in black. A 560 bp long deletion was induced around the TSS of bsAS. A small deletion of 2 nucleotides at the 5’ region of the TSS is also observed. (B) Relative expression of bs and bsAS in bsAS -/- and wt L3 (upper panel) and LP (lower panel) wings. The lncRNA is not expressed in bsAS -/- wings. The overall expression of the bs gene seems to be slightly higher in mutant than in wt wings, especially in L3 stage; however, when comparing the expression of bs isoforms, we do not observe differences in the expression of the short one between the mutant and the wt, while the long isoforms are overexpressed up to 6-fold in the mutants. Error bars depict Standard Error of the Mean (SEM) of at least three biological replicates. Statistical significance was computed by one-sample t-test. (C) Expression pattern of DSRF in bsAS mutant wing imaginal discs. Immunostaining of DSRF (red and grey) in wt (upper panels), bsAS -/+ (middle panels) and bsAS -/- (lower panels) WL3. Nuclei, stained with DAPI, are depicted in blue in all cases. (D) Quantification of DSRF staining in vein and intervein regions of wt and bsAS mutant wing imaginal discs. Both vein and intervein regions exhibit a significant increase of DSRF staining in bsAS mutants compare to wt wings. Statistical significance was computed by two-sample t-test. (E-J) Adult wings from males. (E) Wild type. (F) bsAS heterozygous mutant. The wings present extra veins invading intervein tissue. (G) bsAS homozygous mutant. The wings are creased and vein pattern is completely impaired. (H) bsAS homozygous mutant overexpressing transgenic bsAS under the control of rotund (rn) driver. The mutant phenotype is not rescued by the overexpression of the lncRNA. (I) Wings overexpressing bs isoform A under the control of the rn driver. Extra veins appear within intervein tissue, resembling the phenotype of bsAS heterozygous mutants. (J) bsAS heterozygous mutant wings overexpressing the RNAi against bs long isoform. The overexpression of the RNAi rescues the extra vein phenotype in bsAS heterozygous mutant background.
Ectopic expression of bsAS did not rescue the wing phenotype in the mutant (Figs 2H and S3C), suggesting a role for bsAS in cis, likely due to the transcription process rather than to the bsAS transcript itself. We further observed that the extra vein phenotype is a consequence of the increase in expression of the long isoforms of bs in the bsAS mutant, since similar wing defects were observed when overexpressing the long isoform of bs in the wing pouch, even though the levels of the overexpressed protein were lower than in the heterozygous bsAS mutant (Figs 2I, S3D and S3E). The overexpression of an interference RNA targeting the long isoforms of bs was able to rescue the wild type vein patterning in a heterozygous bsAS mutant background and partially rescue the bsAS homozygous phenotype (Figs 2J and S3F), further confirming the role of the long isoform of bs in promoting vein differentiation. Overexpression of the short isoform B, on the other hand, induces strong defects in wing development and even animal death (S3G and S3H Fig), suggesting that the short DSRF protein is toxic when expressed in excess or in tissues where it is not normally expressed.
bsAS deletion induces overexpression of neural genes
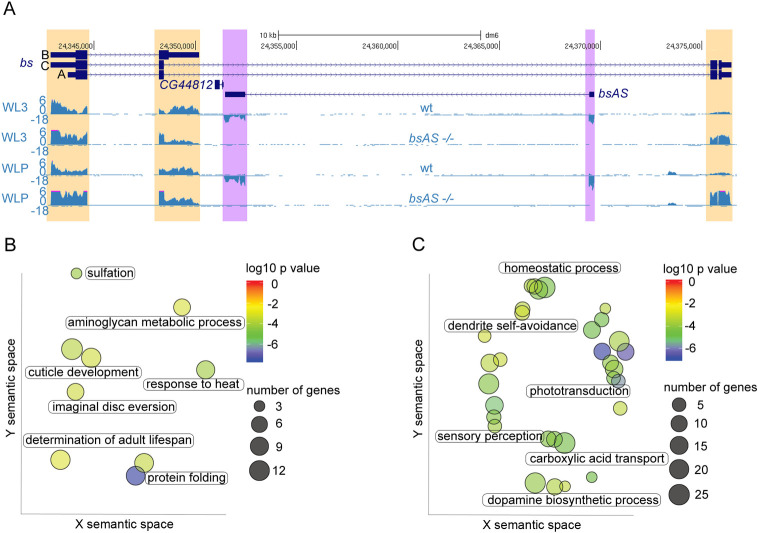
To monitor the molecular changes underlying the bsAS mutant phenotype, we performed RNA-Seq of mutant wing imaginal discs in L3 and LP (Fig 3A and S2 Table). RNA-Seq confirmed the targeted expression results in Fig 2B, and revealed that the long isoform preferentially induced in the bsAS mutant is isoform C, while the expression of isoform A is less affected. We observed few genes differentially expressed comparing the mutant with the wt flies in L3 (27 up and 29 down regulated), but many more in LP: 275 upregulated and 260 downregulated genes (S3 Table). There were not clear functional categories enriched among downregulated genes (Fig 3B) but, after closer inspection, we identified many genes involved in wing morphogenesis and cell adhesion. These include Sox102F, that regulates the expression of the Wnt pathway and has been related to wing vein development and patterning [52], ImpE2 and ImpE3 that are ecdysone-inducible genes related to imaginal disc eversion [53–55] and multiple edematous wings (mew) and miniature (m), that are genes related to cell adhesion and wing morphogenesis [56,57]. In contrast, upregulated genes were strongly enriched in functions related to photo transduction, dopamine biosynthesis and other neural specific functions (Fig 3C). Among the genes upregulated in mutant wings we found Dscam1 and Dscam4, cell adhesion molecules related to axon guidance and neural development [58,59], and inaC, inaD and ninaE, genes involved in the detection and response to light stimuli (for a review see [60]).
Fig 3. bsAS mutation induces overexpression of neural genes in wing imaginal discs.
(A) Expression of bs locus in wt and bsAS -/- wings at L3 (upper panels) and LP (lower panels) –note that wt tracks coincide with tracks in Fig 1C–. Differences in expression between wt and mutant tissues are highlighted in purple for the lncRNA and in orange for the coding gene. The depletion of bsAS expression after bsAS TSS deletion is confirmed in the RNA-Seq experiments, as well as the consequent overexpression of the long bs isoform C. (B) Gene Ontology Term Enrichment analysis of downregulated genes in bsAS-/- WLPs. Few GO categories were enriched among downregulated genes in bsAS-/- LP wings, among them, cuticle development and imaginal disc eversion. (C) Gene Ontology Term Enrichment analysis of upregulated genes in bsAS-/- WLPs. Upregulated genes are enriched in categories mainly related to neural fates, such as phototransduction, sensory perception and dopamine biosynthesis. In (B) and (C), each spot represents an enriched category and labels are representative of clusters of categories; color of the spots represents the p value of enrichment; size of the spots represents number of genes belonging to each category. Axes represent semantic space from ReviGO [29].
All together, these results lead us to hypothesize that the expression of the bs long isoforms may induce the expression of neural specific genes, and that bsAS prevents the development of veins in the wing intervein regions by both impairing the expression of the long isoform C, and promoting the expression of short isoform B. To assess this hypothesis, we analyzed the expression of the bs locus in vein and intervein regions (S4 Fig). Wing imaginal discs expressing the GFP under the control of the bs driver were disaggregated and GFP positive and negative cells were isolated by flow cytometry. Expression analyses indicate that, indeed, bsAS and bs isoform B are mainly expressed in the intervein region (GFP positive cells), while the long isoforms of bs are expressed at similar levels in veins and in the rest of the imaginal disc, suggesting that they are involved in additional roles a part from intervein differentiation.
bsAS and bs are co-regulated through the interaction of their TSSs
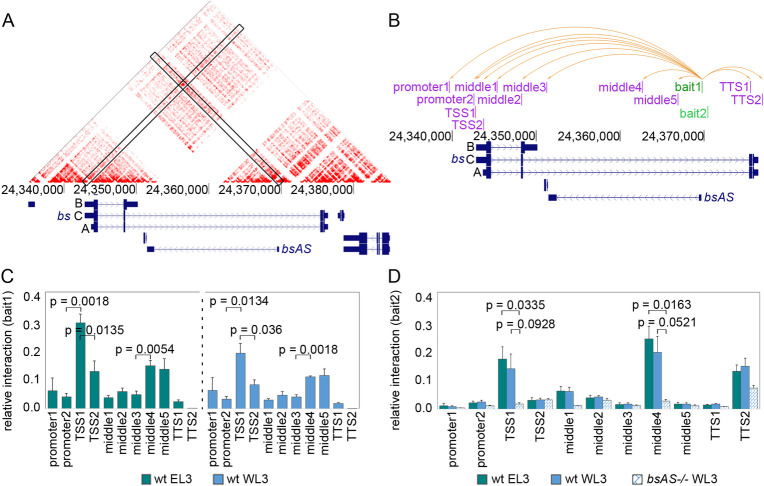
Since bs isoforms B and C share TSS1, we investigated whether physical interactions between the TSS of bsAS and TSS1 could underlay bsAS regulation of bs isoform usage. Using publicly available HiC data in Kc167 cells [61], we did find an enrichment in contacts between the TSS of bsAS and TSS1, even though bs and bsAS are poorly expressed in these cells [62] (Figs 4A and S5A). No other contacts were identified in further regions around the bs locus (S5B Fig). We next performed 3C assays in L3 wing and eye imaginal discs, interrogating the interaction of bsAS TSS with ten different regions along the bs locus (including TSS1 and TSS2) (Fig 4B). We detected a significant interaction with TSS1 both in wing and eye, but not with TSS2, although it is very close to TSS1 (Fig 4C). This interaction seems to be very stable, present even in tissues in which TSS1 and bsAS are inactive, such as Kc197 cells and eye tissue. We detected a second interaction with a region known to be a hot spot for transcription factor binding in fly embryos [63] (S6A Fig). Interactions between TSS1 and a region adjacent to bsAS TSS (bait2), which were maintained in wt wings and eyes, were dramatically impaired in bsAS mutants (Fig 4D), indicating that sequences within this region are likely responsible for the interaction with TSS1.
Fig 4. bsAS and bs are co-regulated through the interaction of their TSSs.
(A) JuiceBox tool [76] was used to visualize high-resolution HiC maps generated in Kc167 cells [61]. A strong interaction signal is observed in the intersection between bs TSS1 and bsAS TSS. (B) Representation of bs locus and the tested interactions inside this region. Two different baits were anchored at bsAS TSS (in green) and interaction enrichment was assessed for each bait by 3C experiments against all depicted regions (in pink). Orange arrows represent all tested interactions for bait1. The same interactions were tested for bait2. (C) Interaction between bait1 and bs locus in wild type eyes (left panel) and wings (right panel). Interaction enrichment is significantly higher between bsAS TSS and TSS1 than between the bsAS TSS and the other tested regions, both in wings and eyes. A significant increase in relative interaction is also detected between bsAS TSS and middle regions 4 and 5. (D) Interaction between bait2 and bs locus in wild type wings and eyes and bsAS-/- wings. The interaction between bait2 and TSS1 is still enriched in wild type tissues, but it is heavily impaired in bsAS mutant wings. Also the interaction between bsAS TSS and middle4 region is lost in the mutant background. In (C) and (D), error bars represent the SEM of at least three biological replicates. Statistical significance was computed by two-tailed t-test.
The only factor for which binding sites were found both at the TSS1 and bsAS TSS was the GAGA factor (GAF), for which no binding sites were found at TSS2. GAF ChIP-Seq data available for L3 wing imaginal discs [31] revealed strong GAF peaks at TSS1 and bsAS TSS (S6B Fig), providing additional support for a role of GAF in mediating the interaction. We also observed two strong Pol II peaks [32] at both TSSs in L3 wings, confirming that the two sites are transcriptionally active in this tissue at this developmental stage.
Evolutionary conservation of bs and bsAS
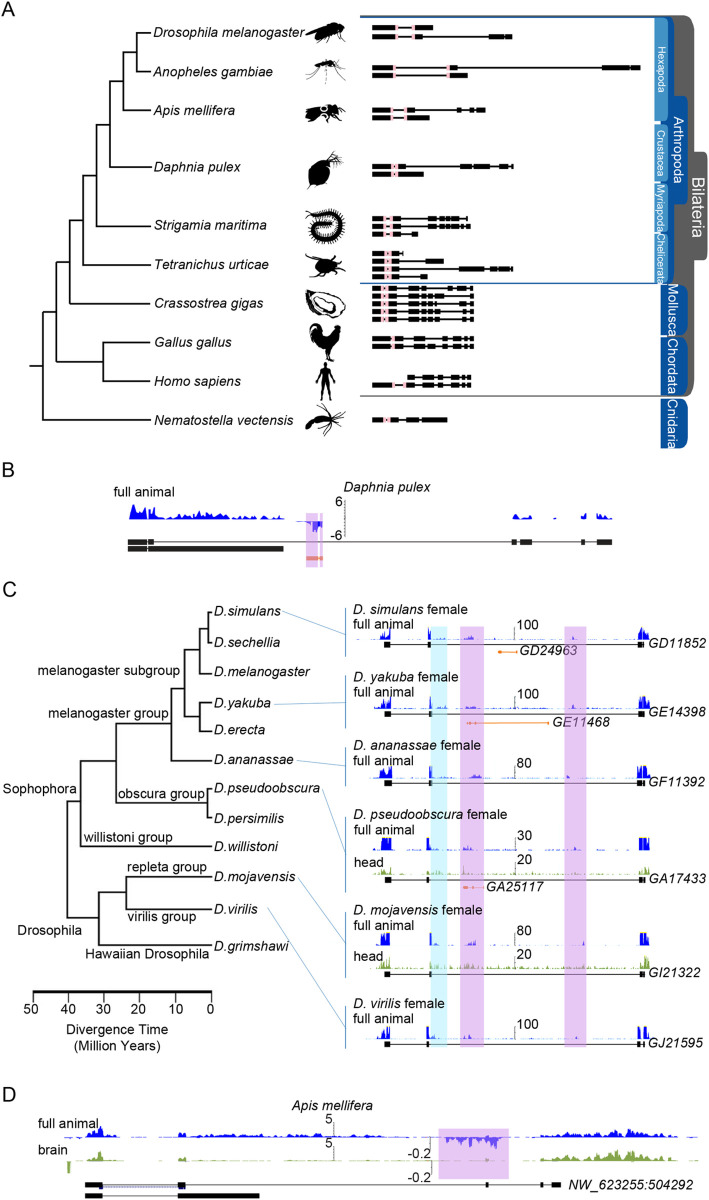
We did not find any known amino acid motif in the 111-aa long region specific to the DSRF long isoform that could explain its specific function in the eye and in the vein regions of the wing. Actually, outside of the MADS box, which is strongly conserved across metazoans (and, intriguingly, systematically interrupted by an intron, in spite of dramatic changes in the exonic structure of the bs gene through evolution), there is little conservation of the DSRF protein, even already within diptera (Figs 5 and S7). By analyzing the existing gene annotation of the investigated species, which we have manually extended using RNA-Seq and computational evidence (see Material and Methods), we found, however, that the long-short isoform structure of bs gene appeared much earlier, at the root of pancrustacea (hexapoda and crustacea, Fig 5A).
Fig 5. Evolutionary conservation of bs and bsAS.
(A) Annotation of bs isoforms along metazoans. bs orthologous genes were manually annotated taking advantage of available RNA-Seq data in all species depicted. The pink box represents the MADS box, conserved along evolution. (B) Expression of bs and bsAS in Daphnia pulex. An antisense transcript is detected within the long intron of bs gene. (C) Expression of bs and bsAS in Drosophila species. Unstranded RNA-Seq tracks from modENCODE project are shown. When available, whole animals (in blue) and heads (in green) RNA-Seq samples have been represented. The long isoform of bs is annotated in all species. We have been able to identify reads likely corresponding to the short isoform of bs (highlighted in blue). D. simulans, D. yakuba and D. pseudoobscura also present annotated antisense genes embedded into the long intron of the coding gene (in orange). In all species, reads corresponding to the bsAS gene have been identified (in purple). (D) Expression of bs and bsAS in A. mellifera whole animals (in blue) and brains (in green). As in fly, bs and bsAS are less expressed in brains compared to whole animals.
Tracking the evolution of bsAS is far more complicated, since lncRNA generally show poor sequence conservation, even at close phylogenetic distances [64,65]. We have, therefore, used RNA-Seq data available across metazoans to characterize antisense transcription within the intron downstream the MADS box of bs gene. We have been able to trace the origin of bsAS also at the root of pancrustacea (Figs 5B and S7B), suggesting that both the long-short isoform structure of bs and bsAS emerged simultaneously. RNA-Seq data available from adult heads in D. mojavensis and D. pseudobscura, as well as from worker bees show that both bs and bsAS are less expressed in heads than in whole animals (Fig 5C and 5D), mirroring our findings in D. melanogaster. All these observations suggest that bsAS has appeared simultaneously to the long-short isoform structure of bs, possibly as a mechanism to regulate isoform usage.
Discussion
Blistered encodes for DSRF (Drosophila Serum Response Factor), orthologous to the SRF protein in humans. In mammalian, SRF is a transcription factor involved in muscle and neural development [17,18]. In flies, bs has been studied both in the context of wing development and in memory establishment and persistence in adult flies [13–16]. In the fruit fly, two wing-specific enhancers are known to regulate bs expression [66], and it has been reported that bs expression is regulated in the wing by the EGF pathway, which negatively regulates its expression in the vein regions [14] and by HH pathway [66] and Vestigial and Scalloped proteins [67], which activate its expression in the wing pouch. However, to date, the regulation of the expression of the bs locus and how this regulation impacts bs function has not been fully elucidated. Here, we show that the expression of the NAT bsAS, one of the most highly expressed lncRNA in the fly wing, plays a crucial role in this regulation. The transcription of bsAS represses the expression of the long isoform, promoting, in consequence, the relative expression of the short isoform. Since the expression of bsAS seems also to prevent the activation of neural genes, we hypothesize that the expression of the long isoforms of bs may contribute to tissue neuralization, while the activation of the short isoform would prevent tissue neuralization.
More specifically, we have found that the bs locus is under the control of two different promoters: TSS1, driving the expression of both the short isoform B and the long isoform C, and interacting with the TSS of bsAS, and TSS2 driving the expression of the long isoform A only. Our results strongly suggest that TSS2 is active in the eye, where long isoform A is expressed. While TSS2 may also be active in the wing, our results suggest that TSS1 is the main driver of bs expression in this tissue, since long isoform C is much more abundant than isoform A. The wing is composed of both non-neural (interveins) and neural tissue (the nerves running along the veins [67]), and we have found the pattern of expression of the bs locus to be quite different in these two regions (S4 Fig). In interveins, the antisense bsAS, the promoter of which interacts with TSS1, is very highly expressed; this impairs the expression of the long isoforms (most likely C) and the short isoform B is predominantly expressed. Low expression of the long isoforms together with high expression of the short isoform, which could act as dominant negative further repressing the action of the long isoform, would lead to the differentiation of intervein tissue. In the vein regions, neither bsAS nor bs isoform B are expressed, and bs long isoform is likely to be the dominant one. We do not know, however, whether this is because TSS2 is the main promoter active in the vein regions, and therefore there is production only of the long isoform A, or because even though TSS1 is the main active promoter, there is specific downregulation of the bsAS, which cannot repress the synthesis of the long isoform C. Although the role of the long isoforms of bs during development is still unclear, several observations point to the idea that they may be related to neural development in the fly. First, we observed that bsAS mutants induced overexpression of bs long isoforms and of neural-specific genes in the wing (Fig 3C), promoting the appearance of extra-vein tissue. Second, bs has been reported to play essential roles in memory and learning in flies [15,16]. This fact altogether with the observation that bs long isoforms are the only forms of bs that are expressed in eye tissue (Fig 1C and 1D), suggest that these isoforms are the responsible for the neural functions assigned to bs/DSRF.
We would like to propose a model to explain how bsAS regulates the isoform usage of the bs gene in the wing to promote intervein differentiation (Fig 6). As mentioned above, in wing interveins, bs TSS1 and bsAS TSS are active, thus RNA Polymerases are recruited in both strands. TSS1 triggers the transcription of both the short isoform B and the long isoform C, but the elongation along both strands provokes the eventual collision of the polymerases and, in consequence, impairs the transcription of long isoform C. Thus, the short isoform B is the dominantly expressed in wing interveins. A collision model was previously suggested as responsible for sense-antisense gene regulation (for a review see [68]). The fact that the long isoforms are also detected in the interveins, albeit at low levels, may respond to a certain permissiveness of the collision. In contrast, when TSS2 is active (as in the eye) or bsAS is inactive, the RNA Polymerase is only recruited in the positive strand and the RNA elongation can occur unobstructed, resulting in the expression of the long isoforms of bs.
Fig 6. Regulation of bs isoform by bsAS.
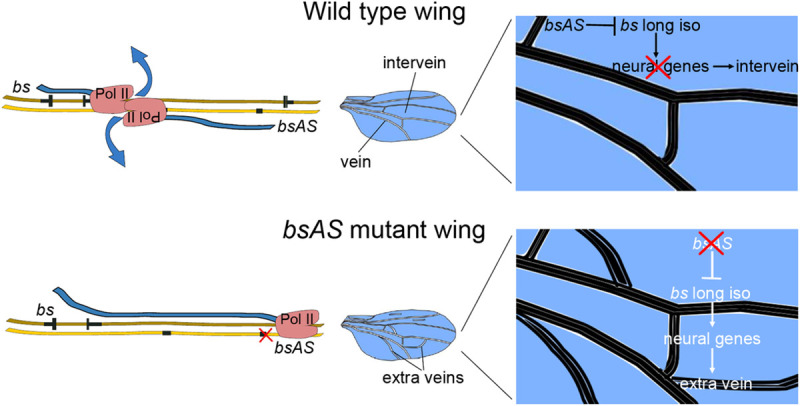
In the upper and bottom panels, positive and negative DNA strands are depicted as dark and light brown ribbons, respectively. Transcribed exons are depicted in black, narrow bars represent non-coding regions and wide bars represent coding regions. Blue ribbons represent nascent RNA. Upper panel. In wild type tissues, the transcription of bsAS inhibits the expression of bs long isoforms in the intervein regions, likely due to the collision of the RNA Polymerases elongating along both strands, which results in the short isoform being the predominant. Lower panel. In bsAS mutant wing intervein regions, as well as in eye tissues, the RNA Polymerase II is recruited only in the positive strand, allowing for the elongation until the end of the locus, and the long isoforms are fully transcribed. The expression of the bs long isoforms in intervein tissue induces the ectopic expression of neural genes and the appearance of extra veins in these regions.
Nevertheless, it is difficult to hypothesize the mechanism by which the different DSRF protein isoforms impact cell fate, since we have not been able to find any known protein domain in the amino acid sequence specific to the long isoform. The only known domain in DSRF is a MADS box domain [13,69], also encoded in human SRF, and present in both isoforms.
The co-expression of bs isoform B and bsAS in the wing pouch prompted us to hypothesize a co-regulated expression of both genes. In this regard, we identified GAF as a potential mediator of both the contact and the co-regulation of bs TSS1 and bsAS TSS. GAF is a transcription factor known to form homodimers and also to interact with the RNA Polymerase machinery, insulator factors and chromatin remodelers [70]. Thus, we hypothesize that the interaction between bs TSS1 and bsAS TSS could be driven by GAF, either by dimerization of GAF proteins binding at the two TSSs or by its interaction with other factors. The deletion of bsAS TSS in bsAS-/- flies, that impairs the interaction between bs and bsAS promoters, includes the GAF binding site found at bsAS promoter region, indicating that this could be, indeed, the factor mediating the contact between both regions. GAF would then act as a transcriptional activator for both bs and bsAS in the intervein region of the wing. Indeed, the downregulation of the Trithorax-like (Trl) gene, encoding for GAF, induces a reduction of the intervein regions [71] and the appearance of extra vein tissue in adult wings [72], confirming a role of GAF in wing development. The loop formed by this interaction is likely stabilized by cohesin complexes, which have been demonstrated to contribute to interactions between chromatin regions such as enhancer-promoter communication [73].
Finally, our results suggest that bsAS has appeared simultaneously to the long-short isoform structure of bs, at the root of pancrustacea, a taxon that includes hexapods and crustaceans. Since non-insect hexapods and crustaceans are wingless animals, bsAS expression is ancestral to the development of the wings in insects. We hypothesize that, given that the neural vs non-neural expression pattern of bsAS is conserved at least within diptera and hymenoptera, bsAS regulation of isoform usage may be originally related to the differentiation of neural and non-neural structures in these animals. Alternatively, bs has been also related to the development of the tracheal system in the fly ([69,74]). Given that wings seem to have evolved from ancestral gills [75], which also have a respiratory function, one could hypothesize that the dual role of bs and its regulation by bsAS can also originate in the development of the tracheal system.
Supporting information
(A) Third instar larvae tissues used for RNA-Seq experiments. (B) Relationship between the correlation of exon ratio and antisense expression and the standard deviation (st. dev.) of sense gene exons ratio. Of the 145 SA pairs with both genes expressed in at least one tissue in L3, 104 pairs (72%, 102 genes) involve a protein coding gene with multiple isoforms. We computed the correlation between the expression of these NATs and the inclusion of the 964 exons of the sense protein coding genes. Because of the very small number of independent data points, we have little power to detect significant correlations. Still, we plotted this correlation against the standard deviation of the exon inclusion values, to focus specifically in the exons that changed the most (see Material and Methods). Among them, the case of blistered -bs- is the strongest, as it indeed shows strong positive and negative correlation between the expression of the NAT antisense to bs and the inclusion of three highly variable bs exons. (C) Ranking of expression of antisense lncRNAs in L3 (upper panel) and LP (lower panel) wings. bsAS is highlighted in red. (D) Histone methylation marks [77] at bs locus. There is a strong peak of H3K4me3 at bs TSS and bsAS TSS in WL3, whereas only a small peak at bs TSS is observed in EAL3. (E) H3K4me3 ChIP-qPCR in WL3. No differences are observed between bs TSS1 and TSS2 H3K4me3 marking. The deletion of bsAS TSS induces a dramatic reduction of H3K4me3 marking in bsAS TSS. TSS1 and TSS2 of bs do not show differences between bsAS-/- and wt. RpL32 and CG5367 correspond to positive and negative controls of the ChIP, respectively.
(TIF)
(A) Drosophila embryos expressing Cas9 nuclease in the germinal cells under the control of vasa driver, were injected with a mix of two plasmids expressing gRNAs against the TSS of bsAS. The screening was performed retroactively, by allowing putatively mutant flies to lie eggs before screening. Once the mutation was isolated, genetic crosses were performed to obtain the homozygous mutant flies. (B) PCR screening of bsAS deletion. A band of 800 bp was amplified in wt flies, whereas a band of 250 bp was obtained from homozygous bsAS mutant flies. (C) Alignment of wt and bsAS-/- genomic regions. A Single Nucleotide Polymorphism was detected in the bsAS TSS sequence (in green). The 5’ region of the deletion has been cleanly repaired, but in the 3’ region, several insertions/deletions have occurred (the insertion of 4 nucleotides–in blue-, the duplication of 9 nucleotides–in green- and the deletion of 2 nucleotides–in yellow-).
(TIF)
(A) Expression pattern of DSRF in bsAS mutant eye imaginal discs. Immunostaining of DSRF (red and grey) in wt (upper panels), bsAS -/+ (middle panels) and bsAS -/- (lower panels) EL3. DSRF is overexpressed in a subset of cells posterior to the morphogenetic furrow in homozygous bsAS mutants. (B) Eyes of w1118 (left panel) and bsAS -/- (right panel) adult males. No evident phenotype is observed in adult eyes, despite the overexpression of DSRF in third instar larvae. (C) Overexpression of bsAS under the control of rotund (rn) driver (specific driver that induces GAL4 expression in the wing pouch) in a bsAS mutant background, checked by qPCR. The overexpression of bsAS does not change the expression of any of bs isoforms. (D) DSRF staining on third instar larvae wings overexpressing the long isoform of bs under the control of rn driver. The overexpression of DSRF protein in L3 wings is significantly lower in wings overexpressing the long isoform of bs than in bsAS mutants. (E-H) Adult wings from males. (E) Wings overexpressing bs long isoform A under the control of the nub driver. They present extra vein tissue. (F) Wings overexpressing RNAi specific against the long isoform of bs in a bsAS homozygous mutant background. They show a partial rescue of the mutant phenotype, being less creased (compare to Fig 2G). (G) Wings expressing the short isoform of bs under the control the salE/Pv driver. They present notches in the wing margin. (H) Wings expressing the short isoform of bs under the control the rn driver. The overexpression of the isoform B of bs induces high lethality and strong impairment of wing development.
(TIF)
(A) Co-localization of GFP and DSRF in bs>GFP heterozygous third instar larvae imaginal discs. (B) Expression of bs isoforms and bsAS in intervein (GFP positive) and vein (GFP negative) regions. bsAS and the bs short isoform B are more expressed in interveins than in veins, which are marked by high expression of the vein-specific genes caupolican (caup) and rhomboid (rho). In contrast, the long isoforms of bs are expressed at comparable levels in veins and interveins. Statistical significance was computed by one-sample t-test.
(TIF)
(A) RNA-Seq of Kc167 cells, from modENCODE [62]. bsAS is not expressed in these cells. bs expression is very low and isoform A seems to be the main expressed one. (B) Interaction of the bs locus and the neighboring regions (100 Kb). HiC data was obtained from Cubenas-Potts et al. [61]. The only strong interaction observed in this region is the contact between bsAS TSS and bs TSS1. No further interactions are observed.
(TIF)
(A) Profile of DNA binding of 24 transcription factors during embryogenesis from modENCODE project [63]. Middle4 region is very close to a hot spot for transcription factor binding. (B) Profile of DNA binding of GAF [31] (light green) and RNA Pol II [32] (dark green) in third instar larvae wings. GAF and RNA Pol II peaks coincide with the expression of bs TSS1 and bsAS in wings. Blue vertical lines represent GAF binding sites at bs TSS1 and bsAS TSS.
(TIF)
(A) Multiple sequence alignment of the long bs isoform using MAFFT and visualization of the frequency-based difference using NCBI MSA Viewer. High sequence conservation is observed between 160–270 bp where MADS-box is located. Sequence conservation drops rapidly outside this region. (B) Read-depth along bs locus. Organisms are sorted by the tree of life. Number of split reads are highlighted in the exon junctions generated using ggsashimi [39]. Stranded RNA-Seq is shown in two separated strands, where the negative strand is negated and shown below the positive strand. When available, whole animals (in blue) and heads (in green) RNA-Seq samples have been represented. The long isoform of bs is annotated in all species. Expression of both long and short isoforms of bs is supported by read coverage and by split alignments in exon junctions in represented species. Antisense expression is supported by stranded read coverage and by split alignments in the exon boundaries in the Diptera Drosophila melanogaster, in the Coleoptera Anoplophora glabripennis and in the Crustacea Daphnia pulex. Stranded RNA-Seq of the Hymenoptera Apis mellifera presents more antisense signal in the whole body compared to the head sample, however there are no split reads supporting exon junctions. The wingless basal Hexapoda, Folsomia candida, presents a clear set of split reads supporting the junctions of bsAS and no split reads shared between bs and bsAS, however the data is unstranded. Overall, Hexapoda and Crustacea show expression of both long-short bs isoforms as well as the antisense expression on the lncRNA bsAS.
(TIF)
All samples generated along the manuscript are summarized here.
(PDF)
WL3, third instar larvae wing. WLP, late pupa wing. EAL3, third instar larvae eye-antenna. ELP, late pupa eye. Genes are highlighted in grey. Expression values are represented in TPMs.
(PDF)
Expression values are represented in TPMs. L3 DEG are represented in sheet 1 and LP DEG are represented in sheet 2.
(XLSX)
Name of the primer, sequence and experiment in which it has been used are depicted.
(XLSX)
Acknowledgments
We thank Bruna R. Correa, Ramil Nurtdinov, Sebastian Ullrich and Beatrice Borsari for helpful discussions about the data and the manuscript and Romina Garrido for administrative assistance. We also thank the Ultrasequencing, the Flow Cytometry and the Advanced Light Microscopy Units of the CRG (Barcelona, Spain), for sample processing and the Rainbow Transgenic Flies Inc. (Camarillo, USA) and the Drosophila Injection Service from the Institut de Recerca Biomèdica (IRB) (Barcelona, Spain) for injection services. We also thank Montserrat Corominas and Florenci Serras, from the University of Barcelona, for the anti-DSRF antibody and for Drosophila strains and Fátima Gebauer, from the Center for Genomic Regulation, for Drosophila strains. This research reflects only the authors’ views and the Community is not liable for any use that may be made of the information contained therein.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. RNA-Seq raw data and processed files were deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-7653.
Funding Statement
This work was supported by the European Community under the FP7 program (ERC-2011-AdG-294653-RNA-MAPS to R.G.), by the Spanish Ministry of Economy and Competitiveness (MEC) (BIO2011-26205 to R.G), by the Centro de Excelencia Severo Ochoa, from the CERCA Programme (Generalitat de Catalunya), and from the Spanish Ministry of Science and Innovation to the EMBL partnership. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Werner A, Sayer JA. Naturally occurring antisense RNA: function and mechanisms of action. Curr Opin Nephrol Hypertens. 2009;18: 343–349. 10.1097/MNH.0b013e32832cb982 [DOI] [PubMed] [Google Scholar]
- 2.Faghihi MA, Wahlestedt C. Regulatory roles of natural antisense transcripts. Nat Rev Mol Cell Biol. 2009;10: 637–643. 10.1038/nrm2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14: 880–893. 10.1038/nrg3594 [DOI] [PubMed] [Google Scholar]
- 4.Khorkova O, Myers AJ, Hsiao J, Wahlestedt C. Natural antisense transcripts. Hum Mol Genet. 2014;23: R54–63. 10.1093/hmg/ddu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrissy AS, Griffith M, Marra MA. Extensive relationship between antisense transcription and alternative splicing in the human genome. Genome Res. 2011;21: 1203–1212. 10.1101/gr.113431.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltran M, Puig I, Pena C, Garcia JM, Alvarez AB, Pena R, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22: 756–769. 10.1101/gad.455708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attrill H, Falls K, Goodman JL, Millburn GH, Antonazzo G, Rey AJ, et al. FlyBase: establishing a Gene Group resource for Drosophila melanogaster. Nucleic Acids Res. 2016;44: D786–792. 10.1093/nar/gkv1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilik I, Akhtar A. roX RNAs: non-coding regulators of the male X chromosome in flies. RNA Biol. 2009;6: 113–121. 10.4161/rna.6.2.8060 [DOI] [PubMed] [Google Scholar]
- 9.Maeda RK, Sitnik JL, Frei Y, Prince E, Gligorov D, Wolfner MF, et al. The lncRNA male-specific abdominal plays a critical role in Drosophila accessory gland development and male fertility. PLoS Genet. 2018;14: e1007519 10.1371/journal.pgen.1007519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz-Losada M, Blom-Dahl D, Cordoba S, Estella C. Specification and Patterning of Drosophila Appendages. J Dev Biol. 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Bellido A, de Celis JF. Developmental genetics of the venation pattern of Drosophila. Annu Rev Genet. 1992;26: 277–304. 10.1146/annurev.ge.26.120192.001425 [DOI] [PubMed] [Google Scholar]
- 12.Fristrom D, Gotwals P, Eaton S, Kornberg TB, Sturtevant M, Bier E, et al. Blistered: a gene required for vein/intervein formation in wings of Drosophila. Development. 1994;120: 2661–2671. [DOI] [PubMed] [Google Scholar]
- 13.Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, Affolter M. The Drosophila Serum Response Factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development. 1996;122: 2589–2597. [DOI] [PubMed] [Google Scholar]
- 14.Roch F, Baonza A, Martin-Blanco E, Garcia-Bellido A. Genetic interactions and cell behaviour in blistered mutants during proliferation and differentiation of the Drosophila wing. Development. 1998;125: 1823–1832. [DOI] [PubMed] [Google Scholar]
- 15.Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science. 2009;324: 105–108. 10.1126/science.1166657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thran J, Poeck B, Strauss R. Serum response factor-mediated gene regulation in a Drosophila visual working memory. Curr Biol. 2013;23: 1756–1763. 10.1016/j.cub.2013.07.034 [DOI] [PubMed] [Google Scholar]
- 17.Coletti D, Daou N, Hassani M, Li Z, Parlakian A. Serum Response Factor in Muscle Tissues: From Development to Ageing. Eur J Transl Myol. 2016;26: 6008 10.4081/ejtm.2016.6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benito E, Barco A. The neuronal activity-driven transcriptome. Mol Neurobiol. 2015;51: 1071–1088. 10.1007/s12035-014-8772-z [DOI] [PubMed] [Google Scholar]
- 19.Perez-Lluch S, Blanco E, Carbonell A, Raha D, Snyder M, Serras F, et al. Genome-wide chromatin occupancy analysis reveals a role for ASH2 in transcriptional pausing. Nucleic Acids Res. 2011;39: 4628–4639. 10.1093/nar/gkq1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, et al. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194: 1029–1035. 10.1534/genetics.113.152710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naumova N, Smith EM, Zhan Y, Dekker J. Analysis of long-range chromatin interactions using Chromosome Conformation Capture. Methods. 2012;58: 192–203. 10.1016/j.ymeth.2012.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.dos Santos G, Schroeder AJ, Goodman JL, Strelets VB, Crosby MA, Thurmond J, et al. FlyBase: introduction of the Drosophila melanogaster Release 6 reference genome assembly and large-scale migration of genome annotations. Nucleic Acids Res. 2015;43: D690–697. 10.1093/nar/gku1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29: 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12: 323 10.1186/1471-2105-12-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raney BJ, Dreszer TR, Barber GP, Clawson H, Fujita PA, Wang T, et al. Track data hubs enable visualization of user-defined genome-wide annotations on the UCSC Genome Browser. Bioinformatics. 2014;30: 1003–1005. 10.1093/bioinformatics/btt637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pohl A, Beato M. bwtool: a tool for bigWig files. Bioinformatics. 2014;30: 1618–1619. 10.1093/bioinformatics/btu056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26: 139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics. 2007;23: 257–258. 10.1093/bioinformatics/btl567 [DOI] [PubMed] [Google Scholar]
- 29.Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6: e21800 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31: 3651–3653. 10.1093/nar/gkg605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh H, Slattery M, Ma L, Crofts A, White KP, Mann RS, et al. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 2013;3: 309–318. 10.1016/j.celrep.2013.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schertel C, Albarca M, Rockel-Bauer C, Kelley NW, Bischof J, Hens K, et al. A large-scale, in vivo transcription factor screen defines bivalent chromatin as a key property of regulatory factors mediating Drosophila wing development. Genome Res. 2015;25: 514–523. 10.1101/gr.181305.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loubiere V, Delest A, Thomas A, Bonev B, Schuettengruber B, Sati S, et al. Coordinate redeployment of PRC1 proteins suppresses tumor formation during Drosophila development. Nat Genet. 2016;48: 1436–1442. 10.1038/ng.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marco-Sola S, Sammeth M, Guigo R, Ribeca P. The GEM mapper: fast, accurate and versatile alignment by filtration. Nat Methods. 2012;9: 1185–1188. 10.1038/nmeth.2221 [DOI] [PubMed] [Google Scholar]
- 35.Davis CA, Hitz BC, Sloan CA, Chan ET, Davidson JM, Gabdank I, et al. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 2018;46: D794–D801. 10.1093/nar/gkx1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen ZX, Sturgill D, Qu J, Jiang H, Park S, Boley N, et al. Comparative validation of the D. melanogaster modENCODE transcriptome annotation. Genome Res. 2014;24: 1209–1223. 10.1101/gr.159384.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao W, Schuler MA, Berenbaum MR. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). Proc Natl Acad Sci U S A. 2017;114: 2538–2543. 10.1073/pnas.1614864114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shpigler HY, Saul MC, Corona F, Block L, Cash Ahmed A, Zhao SD, et al. Deep evolutionary conservation of autism-related genes. Proc Natl Acad Sci U S A. 2017;114: 9653–9658. 10.1073/pnas.1708127114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garrido-Martin D, Palumbo E, Guigo R, Breschi A. ggsashimi: Sashimi plot revised for browser- and annotation-independent splicing visualization. PLoS Comput Biol. 2018;14: e1006360 10.1371/journal.pcbi.1006360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vannini L, Augustine Dunn W, Reed TW, Willis JH. Changes in transcript abundance for cuticular proteins and other genes three hours after a blood meal in Anopheles gambiae. Insect Biochem Mol Biol. 2014;44: 33–43. 10.1016/j.ibmb.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez-Diaz E, Rivero A, Chandre F, Corces VG. Insights into the epigenomic landscape of the human malaria vector Anopheles gambiae. Front Genet. 2014;5: 277 10.3389/fgene.2014.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron RC, Duncan EJ, Dearden PK. Biased gene expression in early honeybee larval development. BMC Genomics. 2013;14: 903 10.1186/1471-2164-14-903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galbraith DA, Yang X, Nino EL, Yi S, Grozinger C. Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog. 2015;11: e1004713 10.1371/journal.ppat.1004713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grbic M, Van Leeuwen T, Clark RM, Rombauts S, Rouze P, Grbic V, et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature. 2011;479: 487–492. 10.1038/nature10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Leeuwen T, Demaeght P, Osborne EJ, Dermauw W, Gohlke S, Nauen R, et al. Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc Natl Acad Sci U S A. 2012;109: 4407–4412. 10.1073/pnas.1200068109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G, Fang X, Guo X, Li L, Luo R, Xu F, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490: 49–54. 10.1038/nature11413 [DOI] [PubMed] [Google Scholar]
- 47.Barbosa-Morais NL, Irimia M, Pan Q, Xiong HY, Gueroussov S, Lee LJ, et al. The evolutionary landscape of alternative splicing in vertebrate species. Science. 2012;338: 1587–1593. 10.1126/science.1230612 [DOI] [PubMed] [Google Scholar]
- 48.Schwaiger M, Schonauer A, Rendeiro AF, Pribitzer C, Schauer A, Gilles AF, et al. Evolutionary conservation of the eumetazoan gene regulatory landscape. Genome Res. 2014;24: 639–650. 10.1101/gr.162529.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frankish A, Diekhans M, Ferreira AM, Johnson R, Jungreis I, Loveland J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lapidot M, Pilpel Y. Genome-wide natural antisense transcription: coupling its regulation to its different regulatory mechanisms. EMBO Rep. 2006;7: 1216–1222. 10.1038/sj.embor.7400857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun M, Hurst LD, Carmichael GG, Chen J. Evidence for variation in abundance of antisense transcripts between multicellular animals but no relationship between antisense transcriptionand organismic complexity. Genome Res. 2006;16: 922–933. 10.1101/gr.5210006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li A, Ahsen OO, Liu JJ, Du C, McKee ML, Yang Y, et al. Silencing of the Drosophila ortholog of SOX5 in heart leads to cardiac dysfunction as detected by optical coherence tomography. Hum Mol Genet. 2013;22: 3798–3806. 10.1093/hmg/ddt230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andres AJ, Fletcher JC, Karim FD, Thummel CS. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev Biol. 1993;160: 388–404. 10.1006/dbio.1993.1315 [DOI] [PubMed] [Google Scholar]
- 54.Moore JT, Fristrom D, Hammonds AS, Fristrom JW. Characterization of IMP-E3, a gene active during imaginal disc morphogenesis in Drosophila melanogaster. Dev Genet. 1990;11: 299–309. 10.1002/dvg.1020110409 [DOI] [PubMed] [Google Scholar]
- 55.Paine-Saunders S, Fristrom D, Fristrom JW. The Drosophila IMP-E2 gene encodes an apically secreted protein expressed during imaginal disc morphogenesis. Dev Biol. 1990;140: 337–351. 10.1016/0012-1606(90)90084-v [DOI] [PubMed] [Google Scholar]
- 56.Brabant MC, Fristrom D, Bunch TA, Brower DL. Distinct spatial and temporal functions for PS integrins during Drosophila wing morphogenesis. Development. 1996;122: 3307–3317. [DOI] [PubMed] [Google Scholar]
- 57.Roch F, Alonso CR, Akam M. Drosophila miniature and dusky encode ZP proteins required for cytoskeletal reorganisation during wing morphogenesis. J Cell Sci. 2003;116: 1199–1207. 10.1242/jcs.00298 [DOI] [PubMed] [Google Scholar]
- 58.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101: 671–684. 10.1016/s0092-8674(00)80878-8 [DOI] [PubMed] [Google Scholar]
- 59.Tadros W, Xu S, Akin O, Yi CH, Shin GJ, Millard SS, et al. Dscam Proteins Direct Dendritic Targeting through Adhesion. Neuron. 2016;89: 480–493. 10.1016/j.neuron.2015.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pak WL, Shino S, Leung HT. PDA (prolonged depolarizing afterpotential)-defective mutants: the story of nina's and ina's—pinta and santa maria, too. J Neurogenet. 2012;26: 216–237. 10.3109/01677063.2011.642430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cubenas-Potts C, Rowley MJ, Lyu X, Li G, Lei EP, Corces VG. Different enhancer classes in Drosophila bind distinct architectural proteins and mediate unique chromatin interactions and 3D architecture. Nucleic Acids Res. 2017;45: 1714–1730. 10.1093/nar/gkw1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duff MO, Olson S, Wei X, Garrett SC, Osman A, Bolisetty M, et al. Genome-wide identification of zero nucleotide recursive splicing in Drosophila. Nature. 2015;521: 376–379. 10.1038/nature14475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Washington NL, Stinson EO, Perry MD, Ruzanov P, Contrino S, Smith R, et al. The modENCODE Data Coordination Center: lessons in harvesting comprehensive experimental details. Database (Oxford). 2011;2011: bar023 10.1093/database/bar023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marques AC, Ponting CP. Catalogues of mammalian long noncoding RNAs: modest conservation and incompleteness. Genome Biol. 2009;10: R124 10.1186/gb-2009-10-11-r124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang KC, Frith MC, Mattick JS. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet. 2006;22: 1–5. 10.1016/j.tig.2005.10.003 [DOI] [PubMed] [Google Scholar]
- 66.Nussbaumer U, Halder G, Groppe J, Affolter M, Montagne J. Expression of the blistered/DSRF gene is controlled by different morphogens during Drosophila trachea and wing development. Mech Dev. 2000;96: 27–36. 10.1016/s0925-4773(00)00373-7 [DOI] [PubMed] [Google Scholar]
- 67.Halder G, Polaczyk P, Kraus ME, Hudson A, Kim J, Laughon A, et al. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 1998;12: 3900–3909. 10.1101/gad.12.24.3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosikiewicz W, Makalowska I. Biological functions of natural antisense transcripts. Acta Biochim Pol. 2016;63: 665–673. 10.18388/abp.2016_1350 [DOI] [PubMed] [Google Scholar]
- 69.Affolter M, Montagne J, Walldorf U, Groppe J, Kloter U, LaRosa M, et al. The Drosophila SRF homolog is expressed in a subset of tracheal cells and maps within a genomic region required for tracheal development. Development. 1994;120: 743–753. [DOI] [PubMed] [Google Scholar]
- 70.Lomaev D, Mikhailova A, Erokhin M, Shaposhnikov AV, Moresco JJ, Blokhina T, et al. The GAGA factor regulatory network: Identification of GAGA factor associated proteins. PLoS One. 2017;12: e0173602 10.1371/journal.pone.0173602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blanch M, Pineyro D, Bernues J. New insights for Drosophila GAGA factor in larvae. R Soc Open Sci. 2015;2: 150011 10.1098/rsos.150011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bejarano F, Busturia A. Function of the Trithorax-like gene during Drosophila development. Dev Biol. 2004;268: 327–341. 10.1016/j.ydbio.2004.01.006 [DOI] [PubMed] [Google Scholar]
- 73.Dorsett D. The Many Roles of Cohesin in Drosophila Gene Transcription. Trends Genet. 2019;35: 542–551. 10.1016/j.tig.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guillemin K, Groppe J, Ducker K, Treisman R, Hafen E, Affolter M, et al. The pruned gene encodes the Drosophila serum response factor and regulates cytoplasmic outgrowth during terminal branching of the tracheal system. Development. 1996;122: 1353–1362. [DOI] [PubMed] [Google Scholar]
- 75.Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385: 627–630. 10.1038/385627a0 [DOI] [PubMed] [Google Scholar]
- 76.Durand NC, Robinson JT, Shamim MS, Machol I, Mesirov JP, Lander ES, et al. Juicebox Provides a Visualization System for Hi-C Contact Maps with Unlimited Zoom. Cell Syst. 2016;3: 99–101. 10.1016/j.cels.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perez-Lluch S, Blanco E, Tilgner H, Curado J, Ruiz-Romero M, Corominas M, et al. Absence of canonical marks of active chromatin in developmentally regulated genes. Nat Genet. 2015;47: 1158–1167. 10.1038/ng.3381 [DOI] [PMC free article] [PubMed] [Google Scholar]