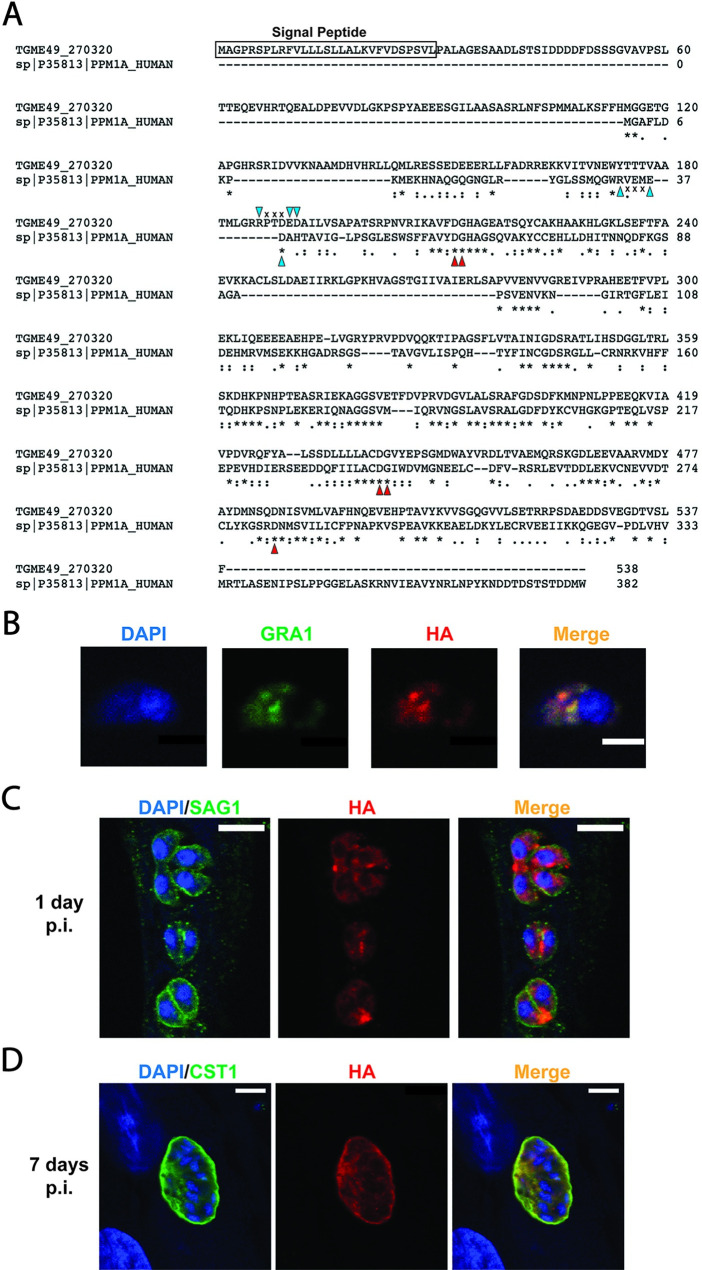
Fig 1. TgPPM3C is a parasitophorous vacuole protein with a PP2C-class phosphatase catalytic domain.
(A) Amino acid sequence alignment of TgPPM3C (Gene ID: TGME49_270320) and human protein phosphatase 1a (PPM1a), a canonical PP2C class phosphatase, performed with Clustal Omega [58]. TgPPM3C contains a predicted signal peptide (boxed region) and an extended N-terminal domain upstream of the conserved PP2C catalytic domain. Residues important for metal binding are indicated with red arrows. Unaligned but conserved residues important for phosphate and metal binding shared by TgPPM3C and PPM1A are indicated by blue arrows. (B) Immunofluorescence image of an extracellular parasite with labeled dense granules (GRA1), endogenously tagged TgPPM3C-HA (HA), and nucleus (DAPI). Partial colocalization is observed between GRA1 and HA, indicating TgPPM3C-HA is potentially packaged into dense granules. Scale bar equals 5μm. (C, D) Immunofluorescence image of parasitophorous vacuoles grown under tachyzoite growth conditions (24 hours post-infection) or bradyzoite growth conditions (D, 7 days post-infection). Parasites in (C) are labeled with SAG1, while bradyzoite differentiation in (D) was probed by glycosylated CST1 expression, detected with SalmonE monoclonal antibody. Vacuolar TgPPM3C is observed more prominently after prolonged culture and parasite development (as in D). Scale bars indicate 10μm.